Stereoselective synthesis of heterocyclic tetraphenylethylene analogues with configuration-dependent solid-state luminescence
- PMID: 40371368
- PMCID: PMC12070306
- DOI: 10.1039/d4sc08333d
Stereoselective synthesis of heterocyclic tetraphenylethylene analogues with configuration-dependent solid-state luminescence
Abstract
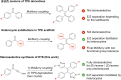
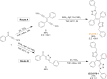
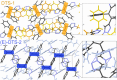
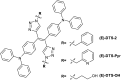
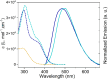
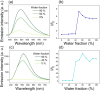
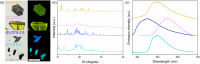
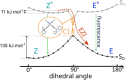
While nowadays ubiquitous in a variety of optoelectronic applications, fluorophores displaying aggregation induced emission (AIE) and in particular those constructed around the tetraphenylethylene (TPE) core suffer severe limitations. In particular, it has been reported in many instances that stereoconfiguration around the central double bond may severely impact the solid-state luminescence properties (maximal emission wavelength and fluorescence quantum yield). Stereoselective synthesis of extended TPE cores remains challenging, and separation of diastereoisomer mixtures is generally tedious. In this paper, we introduce ditriazolostilbene moities (DTS) as an alternative to TPE. DTS offers two significant advantages over its TPE counterpart: firstly, a fully stereoselective synthesis of the (E)-isomer, and secondly, the use of a copper-catalyzed azide-alkyne cycloaddition (CuAAc) reaction in the final step, which simplifies access to novel derivatives. We illustrate the benefits of this approach using stereopure and (E) and (Z)-aggregates, powders and crystals of the molecule and show that emission properties are considerably dependent on their stereoconfiguration.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Two-Photon Absorbing AIEgens: Influence of Stereoconfiguration on Their Crystallinity and Spectroscopic Properties and Applications in Bioimaging.ACS Appl Mater Interfaces. 2020 Dec 9;12(49):55157-55168. doi: 10.1021/acsami.0c15810. Epub 2020 Nov 20. ACS Appl Mater Interfaces. 2020. PMID: 33217234
-
Tetraphenylethylene-Carborane-Tetraphenylethylene Triad: Influence of Steric Bridge on Aggregation-Induced Emission Properties.Chem Asian J. 2018 Nov 2;13(21):3155-3159. doi: 10.1002/asia.201801172. Epub 2018 Sep 26. Chem Asian J. 2018. PMID: 30133156
-
Three Isomeric Tetraphenylethylene-pyridine Compounds: Synthesis, Crystal Structures, and Photophysical Properties.Chem Asian J. 2023 Sep 15;18(18):e202300600. doi: 10.1002/asia.202300600. Epub 2023 Aug 22. Chem Asian J. 2023. PMID: 37561069
-
AIE-Based & Organic Luminescent Materials: Nanoarchitectonics and Advanced Applications.Chem Asian J. 2024 Nov 4;19(21):e202400682. doi: 10.1002/asia.202400682. Epub 2024 Oct 17. Chem Asian J. 2024. PMID: 39136399 Review.
-
Macrocycles and cages based on tetraphenylethylene with aggregation-induced emission effect.Chem Soc Rev. 2018 Oct 1;47(19):7452-7476. doi: 10.1039/c8cs00444g. Chem Soc Rev. 2018. PMID: 30177975 Review.
References
-
- Zhao Z. Lam J. W. Y. Tang B. Z. J. Mater. Chem. 2012;22:23726. doi: 10.1039/C2JM31949G. - DOI
LinkOut - more resources
Full Text Sources

