Challenges of in vitro modelling of liver fibrosis
- PMID: 40371390
- PMCID: PMC12075197
- DOI: 10.3389/fcell.2025.1567916
Challenges of in vitro modelling of liver fibrosis
Abstract
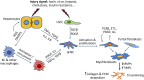
Liver fibrosis has been proposed as the most important predictive indicator affecting prognosis of patients with chronic liver disease. It is defined by an abnormal accumulation of extracellular matrix components that results from necrotic and inflammatory processes and eventually impairs organ function. With no approved therapy, comprehensive cellular models directly derived from patient's cells are necessary to understand the mechanisms behind fibrosis and the response to anti-fibrotic therapies. Primary human cells, human hepatic cell lines and human stem cells-derived hepatic stellate-like cells have been widely used for studying fibrosis pathogenesis. In this paper, we depict the cellular crosstalk and the role of extracellular matrix during fibrosis pathogenesis and summarize different in vitro models from simple monolayers to multicellular 3D cultures used to gain deeper mechanistic understanding of the disease and the therapeutic response, discussing their major advantages and disadvantages for liver fibrosis modelling.
Keywords: 3D models; extracellular matrix; in vitro systems; liver fibrosis; therapies.
Copyright © 2025 Ros-Tarraga, Villanueva-Badenas, Sanchez-Gonzalez, Gallego-Ferrer, Donato and Tolosa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Impact of heterogeneity in liver matrix and intrahepatic cells on the progression of hepatic fibrosis.Tissue Cell. 2024 Dec;91:102559. doi: 10.1016/j.tice.2024.102559. Epub 2024 Sep 12. Tissue Cell. 2024. PMID: 39293139 Review.
-
The fibrotic response of primary liver spheroids recapitulates in vivo hepatic stellate cell activation.Biomaterials. 2020 Dec;261:120335. doi: 10.1016/j.biomaterials.2020.120335. Epub 2020 Aug 22. Biomaterials. 2020. PMID: 32891040
-
Collagenase Type I and Probucol-Loaded Nanoparticles Penetrate the Extracellular Matrix to Target Hepatic Stellate Cells for Hepatic Fibrosis Therapy.Acta Biomater. 2024 Feb;175:262-278. doi: 10.1016/j.actbio.2023.12.027. Epub 2023 Dec 21. Acta Biomater. 2024. PMID: 38141933
-
Dynamics of compartment-specific proteomic landscapes of hepatotoxic and cholestatic models of liver fibrosis.Elife. 2025 Apr 8;13:RP98023. doi: 10.7554/eLife.98023. Elife. 2025. PMID: 40197391 Free PMC article.
References
-
- Angulo P., Kleiner D. E., Dam-Larsen S., Adams L. A., Bjornsson E. S., Charatcharoenwitthaya P., et al. (2015). Liver fibrosis, but No other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149 (2), 389–397. 10.1053/j.gastro.2015.04.043 - DOI - PMC - PubMed
-
- Asadollahi N., Hajari M. A., Alipour Choshali M., Ajoudanian M., Ziai S. A., Vosough M., et al. (2024). Bioengineering scalable and drug-responsive in vitro human multicellular non-alcoholic fatty liver disease microtissues encapsulated in the liver extracellular matrix-derived hydrogel. EXCLI J. 23, 421–440. 10.17179/excli2023-6878 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

