Acid/Base-Responsive Circularly Polarized Luminescence Emitters with Configurationally Stable Nitrogen Stereogenic Centers
- PMID: 40371460
- PMCID: PMC12288784
- DOI: 10.1002/adma.202417326
Acid/Base-Responsive Circularly Polarized Luminescence Emitters with Configurationally Stable Nitrogen Stereogenic Centers
Abstract
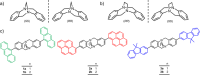
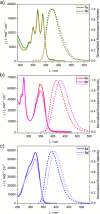
A way to prevent the fast configurational interconversion of tertiary amines is to invoke Tröger's base analogs, which display methano- or ethano-bridged diazocine cores fused to aromatic rings. These derivatives are configurationally stable, even in acidic media when their structures bear ethylene bridges. Here, a two- to three-step synthesis is presented of methano- and ethano-bridged Tröger's base analogs with two peripheral fluorophores, i.e., anthracene, pyrene, and 9,9-dimethylfluorene units. These compounds, possessing two nitrogen stereogenic centers, exhibit good circularly polarized luminescence (CPL) dissymmetry factors (|glum| up to 1.2 × 10-3) and brightnesses (BCPL up to 26.3 M-1 cm-1), as well as excellent fluorescence quantum yields, demonstrating the Tröger´s base core to be a convenient scaffold to prepare CPL emitters upon functionalization with simple achiral fluorophores. Furthermore, the configurationally stable ethano-bridged Tröger's base analogs are employed to modulate their CPL response, generating a CPL switch through their protonation/deprotonation by consecutive additions of acid and base. The reversibility of the switching process is demonstrated for two cycles without altering the CPL performance of the molecule. It is believed that this straightforward and efficient approach to building CPL emitters employing the Tröger's base core could lead to its incorporation in CPL-based sensors and materials.
Keywords: Tröger's Base; chiroptical switch; circularly polarized luminescence; configurationally stable nitrogens; fluorophores.
© 2025 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Circularly Polarized Luminescence Signal Inversion in Aza-[7]Helicene Derivatives: Theoretical Insight into Pyridine Nitrogen Position and Protonation Effects.Chemphyschem. 2025 Jul 18;26(14):e202500106. doi: 10.1002/cphc.202500106. Epub 2025 May 20. Chemphyschem. 2025. PMID: 40289022
-
Modulating circularly polarized luminescent and thermally activated delayed fluorescence properties by introducing chiral unit and extending acceptor unit strategies.Spectrochim Acta A Mol Biomol Spectrosc. 2026 Jan 5;344(Pt 1):126710. doi: 10.1016/j.saa.2025.126710. Epub 2025 Jul 18. Spectrochim Acta A Mol Biomol Spectrosc. 2026. PMID: 40700904
-
Solvent Modulation of Chiral Perovskite Films Enables High Circularly Polarized Luminescence Performance from Chiral Perovskite/Quantum Dot Composites.ACS Appl Mater Interfaces. 2023 Feb 22;15(7):9978-9986. doi: 10.1021/acsami.2c20716. Epub 2023 Feb 8. ACS Appl Mater Interfaces. 2023. PMID: 36753711
-
Advances in nitrogen-containing helicenes: synthesis, chiroptical properties, and optoelectronic applications.Beilstein J Org Chem. 2025 Jul 11;21:1422-1453. doi: 10.3762/bjoc.21.106. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 40661750 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
-
- Lehn J.‐M., in Dynamic Stereochemistry, Vol. 15, (Eds.: Baldwin J. E., Fleming R. H., Lehn J. M., Tochtermann W.), Springer‐Verlag, Berlin: 1970, pp. 311–377.
-
- a) Fröhlich E., Wedekind E., Chem. Ber. 1907, 40, 1009;
- b) Walsh M. P., Phelps J. M., Lennon M. E., Yufit D. S., Kitching M. O., Nature 2021, 597, 70. - PubMed
-
- a) Dolling U.‐H., Davis P., Grabowski E. J. J., J. Am. Chem. Soc. 1984, 106, 446;
- b) O'Donnell M. J., Bennett W. D., Wu S., J. Am. Chem. Soc. 1989, 111, 2353;
- c) Corey E. J., Xu F., Noe M. C., J. Am. Chem. Soc. 1997, 119, 12414;
- d) Corey E. J., Zhang F.‐Y., Org. Lett. 1999, 1, 1287; - PubMed
- e) Cortigiani M., Mereu A., Healy M. G., Adamo M. F. A., J. Org. Chem. 2019, 84, 4112. - PubMed
-
- a) Traverse J. F., Zhao Y., Hoveyda A. H., Snapper M. L., Org. Lett. 2005, 7, 3151; - PubMed
- b) Bhadra S., Yamamoto H., Angew. Chem., Int. Ed. 2016, 55, 13043; - PubMed
- c) Liu R.‐M., Wang Y.‐H., Chen Z.‐Y., Zhang L., Shi Q.‐H., Zhou Y., Tian Y.‐P., Liu X.‐L., Org. Chem. Front. 2022, 9, 6881;
- d) Luo Z., Liao M., Li W., Zhao S., Tang K., Zheng P., Chi Y. R., Zhang X., Wu X., Angew. Chem., Int. Ed. 2024, 63, 202404979. - PubMed
Grants and funding
- PID2020-112906GA-I00/Ministerio de Ciencia, Innovación y Universidades / Agencia Estatal de Investigación
- PID2020-113059GB-C21/Ministerio de Ciencia, Innovación y Universidades / Agencia Estatal de Investigación
- PID2021-127521NB-I00/Ministerio de Ciencia, Innovación y Universidades / Agencia Estatal de Investigación / ERDF, EU
- PID2023-146433NB-I00/Ministerio de Ciencia, Innovación y Universidades / Agencia Estatal de Investigación / ERDF, EU
LinkOut - more resources
Full Text Sources
Miscellaneous

