Seminal vesicle protein caCA12 in Corydoras aeneus inhibits sperm motility for sperm drinking
- PMID: 40371598
- PMCID: PMC12091868
- DOI: 10.1242/jeb.250293
Seminal vesicle protein caCA12 in Corydoras aeneus inhibits sperm motility for sperm drinking
Abstract
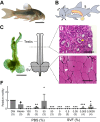
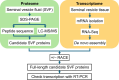
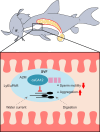
Seminal vesicle (SV) secretions enhance fertilization by regulating sperm motility and fertilization capacity, and by forming plugs that prevent mating with other males. Although SVs are rare in teleosts, certain species, such as Corydoras spp., do possess them. In Corydoras spp. and other species that exhibit sperm drinking or related behaviors, females attach their mouths to the males' genital pore to ingest semen, a reproductive behavior known as sperm drinking. However, the major proteins and functions of seminal vesicle fluid (SVF) in Corydoras remain unidentified. This study aimed to identify the SVF proteins in Corydoras aeneus and clarify the functions of the identified major SVF proteins. The SVF of this species was found to be highly viscous with a high protein concentration. Sperm motility was strongly suppressed in the presence of the SVF. We identified three SVF proteins - alpha-2-macroglobulin (A2M), carbonic anhydrase 12 (CA12) and lymphocyte antigen 6 (Ly6) - through RNA sequencing (RNA-Seq), LC-MS/MS and amino acid sequencing. Additionally, we found that the identified CA12, termed 'caCA12,' was degraded into about 10 kDa and 33 kDa polypeptides containing the CA domain. The 33 kDa polypeptide with the CA domain was found to inhibit sperm motility. The identified SVF proteins, including caCA12, may play a role in keeping sperm in an immotile state until they are close to the female ova, facilitating the remarkable sperm drinking reproductive process observed in C. aeneus.
Keywords: Corydoras; Carbonic anhydrase; Inhibition of sperm motility; Seminal vesicle fluid; Sperm drinking.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


Similar articles
-
Drosophila seminal sex peptide associates with rival as well as own sperm, providing SP function in polyandrous females.Elife. 2020 Jul 16;9:e58322. doi: 10.7554/eLife.58322. Elife. 2020. PMID: 32672537 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Seminal plasma metabolomics and sperm lipidomics profiles of bull semen with different total progressive motile sperm count.J Anim Sci. 2025 Jan 4;103:skaf012. doi: 10.1093/jas/skaf012. J Anim Sci. 2025. PMID: 39887007
-
Antioxidants for male subfertility.Cochrane Database Syst Rev. 2014;(12):CD007411. doi: 10.1002/14651858.CD007411.pub3. Epub 2014 Dec 15. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Mar 14;3:CD007411. doi: 10.1002/14651858.CD007411.pub4. PMID: 25504418 Updated.
-
Improving sperm selection strategies for assisted reproduction through closing the knowledge gap in sperm maturation mechanics.Hum Reprod Open. 2025 Jul 3;2025(3):hoaf040. doi: 10.1093/hropen/hoaf040. eCollection 2025. Hum Reprod Open. 2025. PMID: 40698010 Free PMC article. Review.
References
-
- Chowdhury, I. and Joy, K. P. (2007). Seminal vesicle and its role in the reproduction of teleosts. Fish Physiol. Biochem. 33, 383-398. 10.1007/s10695-007-9162-5 - DOI
-
- Cosson, J., Groison, A. L., Suquet, M., Fauvel, C., Dreanno, C. and Billard, R. (2008). Studying sperm motility in marine fish: an overview on the state of the art. J. Appl. Ichthyol. 24, 460-486. 10.1111/j.1439-0426.2008.01151.x - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

