Rationale and Design of the REPEAT Trial: A Multicenter Randomized Trial Comparing Redo Surgical Aortic Valve Replacement to Valve-in-Valve Transcatheter Aortic Valve Replacement
- PMID: 40371620
- PMCID: PMC12184579
- DOI: 10.1161/JAHA.125.040954
Rationale and Design of the REPEAT Trial: A Multicenter Randomized Trial Comparing Redo Surgical Aortic Valve Replacement to Valve-in-Valve Transcatheter Aortic Valve Replacement
Abstract
Background: Redo surgical aortic valve replacement (rSAVR) has for long been the therapeutic reference standard for degenerated surgical aortic bioprostheses. Valve-in-valve transcatheter aortic valve replacement (ViV-TAVR) has emerged as an alternative for patients at high surgical risk due to its lower invasiveness. The long-term clinical efficacy of ViV-TAVR in patients at low to intermediate surgical risk remains unknown.
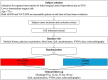
Methods and results: To compare clinical outcomes of redo surgical aortic valve replacement versus ViV-TAVR in low- to intermediate-risk patients with degenerated surgical aortic bioprostheses. REPEAT (Repeat Intervention for Deteriorated Surgical Bioprosthetic Aortic Valves) is an investigator-initiated, international, multicenter, randomized, parallel, open-label trial. A total of 890 patients aged <75 years with a failed surgical aortic bioprosthesis due to structural valve degeneration and low to intermediate surgical risk (ie, Society of Thoracic Surgeons predicted risk of death of <8%) will be randomly assigned in a 1:1 ratio to either redo surgical aortic valve replacement or ViV-TAVR. The primary end point of REPEAT is a composite of all-cause death, stroke (including both disabling and nondisabling), myocardial infarction, and rehospitalization for heart failure or aortic valve reintervention at 5 years, based on Valve Academic Research Consortium-3 definitions. Secondary end points include each of the individual components of the primary composite end point, Valve Academic Research Consortium-3-based conduction disturbances and arrhythmia, Valve Academic Research Consortium-3-based wound and bleeding complications, functional status (ie, 6-minute walk test, Kansas City Cardiomyopathy questionnaire), and treatment costs.
Conclusions: The REPEAT trial has been designed to test the hypothesis that redo surgical aortic valve replacement is superior to ViV-TAVR regarding clinical outcomes at 5 years in patients with degenerated surgical aortic bioprostheses and low to intermediate surgical risk.
Keywords: aortic stenosis; failed surgical aortic bioprosthesis; redo surgical aortic valve replacement; structural valve deterioration; valve‐in‐valve transcatheter aortic valve replacement.
Conflict of interest statement
None.
Figures
References
-
- Ramos J, Monteagudo JM, Gonzalez‐Alujas T, Fuentes ME, Sitges M, Pena ML, Carrasco‐Chinchilla F, Echeverria T, Bouzas A, Forteza Alberti JF, et al. Large‐scale assessment of aortic stenosis: facing the next cardiac epidemic? Eur Heart J Cardiovasc Imaging. 2018;19:1142–1148. doi: 10.1093/ehjci/jex223 - DOI - PubMed
-
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, et al. ACC/AHA guideline for the Management of Patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143:e72–e227. doi: 10.1161/CIR.0000000000000923 - DOI - PubMed
-
- Brown JM, O'Brien SM, Wu C, Sikora JA, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015 - DOI - PubMed
-
- Dunning J, Gao H, Chambers J, Moat N, Murphy G, Pagano D, Ray S, Roxburgh J, Bridgewater B. Aortic valve surgery: marked increases in volume and significant decreases in mechanical valve use‐‐an analysis of 41,227 patients over 5 years from the Society for Cardiothoracic Surgery in Great Britain and Ireland national database. J Thorac Cardiovasc Surg. 2011;142:776–782. doi: 10.1016/j.jtcvs.2011.04.048 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

