Asparagine endopeptidase cleaves apolipoprotein A1 and accelerates pathogenesis of atherosclerosis
- PMID: 40371638
- PMCID: PMC12077905
- DOI: 10.1172/JCI185128
Asparagine endopeptidase cleaves apolipoprotein A1 and accelerates pathogenesis of atherosclerosis
Abstract
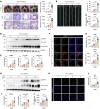
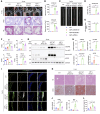
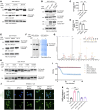
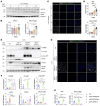
Atherosclerosis is a slowly progressing inflammatory disease characterized with cholesterol disorder and intimal plaques. Asparagine endopeptidase (AEP) is an endolysosomal protease that is activated under acidic conditions and is elevated substantially in both plasma and plaques of patients with atherosclerosis. However, how AEP accelerates atherosclerosis development remains incompletely understood, especially from the view of cholesterol metabolism. This project aims to reveal the crucial substrate of AEP during atherosclerosis plaque formation and to lay the foundation for developing novel therapeutic agents for Atherosclerosis. Here, we show that AEP is augmented in the atherosclerosis plaques obtained from patients and proteolytically cuts apolipoprotein A1 (APOA1) and impairs cholesterol efflux and high-density lipoprotein (HDL) formation, facilitating atherosclerosis pathologies. AEP is activated in the liver and aorta of apolipoprotein E-null (APOE-null) mice, and deletion of AEP from APOE-/- mice attenuates atherosclerosis. APOA1, an essential lipoprotein in HDL for cholesterol efflux, is cleaved by AEP at N208 residue in the liver and atherosclerotic macrophages of APOE-/- mice. Blockade of APOA1 cleavage by AEP via N208A mutation or its specific inhibitor, #11a, substantially diminishes atherosclerosis in both APOE-/- and LDLR-/- mice. Hence, our findings support that AEP disrupts cholesterol metabolism and accelerates the development of atherosclerosis.
Keywords: Atherosclerosis; Cardiology; Cholesterol; Macrophages; Vascular biology.
Conflict of interest statement
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

