AgRP neuron hyperactivity drives hyperglycemia in a mouse model of type 2 diabetes
- PMID: 40371641
- PMCID: PMC12077889
- DOI: 10.1172/JCI189842
AgRP neuron hyperactivity drives hyperglycemia in a mouse model of type 2 diabetes
Abstract
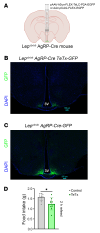
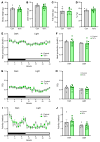
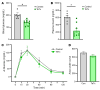
Growing evidence suggests that the pathogenesis of type 2 diabetes (T2D) involves dysfunctional central mechanisms, and, hence, the brain can be targeted to treat this disease. As an example, a single intracerebroventricular (icv) injection of fibroblast growth factor 1 (FGF1) can normalize hyperglycemia for weeks or months in rodent models of T2D. Convergent evidence implicates inhibition of a particular subset of neurons as a mediator of this FGF1 effect. Specifically, AgRP neurons, which are located in the hypothalamic arcuate nucleus (ARC) and are hyperactive in Lepob/ob mice and other rodent models of T2D. To investigate whether chronic AgRP neuron inactivation mimics the antidiabetic action of FGF1, we directed an adeno-associated virus (AAV) containing a cre-inducible tetanus toxin-GFP (TeTx-GFP) cassette (or cre-inducible AAV GFP control) to the ARC of obese, diabetic male Lepob/ob mice in which cre recombinase is expressed solely by AgRP neurons (Lepob/ob AgRP-Cre mice). We report that over a 10-wk period of observation, hyperglycemia was fully normalized by AgRP neuron inactivation. In contrast, changes in energy homeostasis parameters (food intake, energy expenditure, body weight, and fat mass) were not observed. We conclude that in diabetic male Lepob/ob mice, AgRP neuron hyperactivity is required for hyperglycemia but is dispensable for obesity.
Keywords: Diabetes; Endocrinology; Metabolism.
Conflict of interest statement
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

