Clemastine fumarate accelerates accumulation of disability in progressive multiple sclerosis by enhancing pyroptosis
- PMID: 40371642
- PMCID: PMC12077908
- DOI: 10.1172/JCI183941
Clemastine fumarate accelerates accumulation of disability in progressive multiple sclerosis by enhancing pyroptosis
Abstract
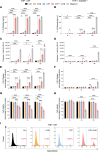
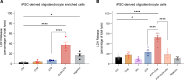
Multiple sclerosis (MS) is an immune-mediated demyelinating disease of the CNS. Clemastine fumarate, the over-the-counter antihistamine and muscarinic receptor blocker, has remyelinating potential in MS. A clemastine arm was added to an ongoing platform clinical trial, targeting residual activity by precision, biomarker-guided combination therapies of multiple sclerosis (TRAP-MS) (ClinicalTrials.gov NCT03109288), to identify a cerebrospinal fluid (CSF) remyelination signature and to collect safety data on clemastine in patients progressing independently of relapse activity (PIRA). The clemastine arm was stopped per protocol-defined criteria when 3 of 9 patients triggered individual safety stopping criteria. Clemastine-treated patients had significantly higher treatment-induced disability progression slopes compared with the remaining TRAP-MS participants. Quantification of approximately 7,000 proteins in CSF samples collected before and after clemastine treatment showed significant increases in purinergic signaling and pyroptosis. Mechanistic studies showed that clemastine with sublytic doses of extracellular adenosine triphosphate (ATP) activates inflammasome and induces pyroptotic cell death in macrophages. Clemastine with ATP also caused pyroptosis of induced pluripotent stem cell-derived human oligodendrocytes. Antagonist of the purinergic channel P2RX7, which is strongly expressed in oligodendrocytes and myeloid cells, blocked these toxic effects of clemastine. Finally, reanalysis of published single-nucleus RNA-Seq (snRNA-Seq) studies revealed increased P2RX7 expression and pyroptosis transcriptional signature in microglia and oligodendrocytes in the MS brain, especially in chronic active lesions. The CSF proteomic pyroptosis score was increased in untreated MS patients, was higher in patients with progressive than relapsing-remitting disease, and correlated significantly with the rates of MS progression. Collectively, this identifies pyroptosis as a likely mechanism of CNS injury underlying PIRA even outside of clemastine toxicity.
Keywords: Cellular immune response; Immunology; Multiple sclerosis; Neurodegeneration; Neuroscience.
Conflict of interest statement
Figures




Update of
-
Clemastine fumarate accelerates accumulation of disability in progressive multiple sclerosis by enhancing pyroptosis.medRxiv [Preprint]. 2024 Apr 10:2024.04.09.24305506. doi: 10.1101/2024.04.09.24305506. medRxiv. 2024. Update in: J Clin Invest. 2025 May 15;135(10):e183941. doi: 10.1172/JCI183941. PMID: 39802756 Free PMC article. Updated. Preprint.
Similar articles
-
Clemastine fumarate accelerates accumulation of disability in progressive multiple sclerosis by enhancing pyroptosis.medRxiv [Preprint]. 2024 Apr 10:2024.04.09.24305506. doi: 10.1101/2024.04.09.24305506. medRxiv. 2024. Update in: J Clin Invest. 2025 May 15;135(10):e183941. doi: 10.1172/JCI183941. PMID: 39802756 Free PMC article. Updated. Preprint.
-
Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial.Lancet. 2017 Dec 2;390(10111):2481-2489. doi: 10.1016/S0140-6736(17)32346-2. Epub 2017 Oct 10. Lancet. 2017. PMID: 29029896 Clinical Trial.
-
Measuring and predicting the effect of remyelinating therapy in multiple sclerosis: a randomised controlled trial protocol (RESTORE).BMJ Open. 2024 Jan 30;14(1):e076651. doi: 10.1136/bmjopen-2023-076651. BMJ Open. 2024. PMID: 38296293 Free PMC article.
-
Enhancing remyelination in multiple sclerosis via M1 muscarinic acetylcholine receptor.Mol Pharmacol. 2025 Apr;107(4):100027. doi: 10.1016/j.molpha.2025.100027. Epub 2025 Feb 28. Mol Pharmacol. 2025. PMID: 40158341 Free PMC article. Review.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
Cited by
-
The potential of repurposing clemastine to promote remyelination.Front Cell Neurosci. 2025 May 7;19:1582902. doi: 10.3389/fncel.2025.1582902. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40400770 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

