TMEM219 signaling promotes intestinal stem cell death and exacerbates colitis
- PMID: 40371646
- PMCID: PMC12077909
- DOI: 10.1172/JCI185783
TMEM219 signaling promotes intestinal stem cell death and exacerbates colitis
Abstract
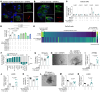
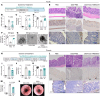
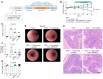
Mechanisms by which mucosal regeneration is abrogated in inflammatory bowel disease (IBD) are still under investigation, and a role for an intestinal stem cell (ISC) defect is now emerging. Herein, we report an abnormal ISC death that occurs in Crohn's disease, which exacerbates colitis, limits ISC-dependent mucosal repair, and is controlled through the death factor Transmembrane protein 219 (TMEM219). Large alterations in TMEM219 expression were observed in patients with Crohn's disease, particularly in those with active disease and/or those who were nonresponders to conventional therapy, confirming that TMEM219 signaling is abnormally activated and leads to failure of the mucosal regenerative response. Mechanistic studies revealed a proapoptotic TMEM219-mediated molecular signature in Crohn's disease, which associates with Caspase-8 activation and ISC death. Pharmacological blockade of the IGFBP3/TMEM219 binding/signal with the recombinant protein ecto-TMEM219 restored the self-renewal abilities of miniguts generated from patients with Crohn's disease in vitro and ameliorated DSS-induced and T cell-mediated colitis in vivo, ultimately leading to mucosal healing. Genetic tissue-specific deletion of TMEM219 in ISCs in newly generated TMEM219fl/flLGR5cre mice revived their mucosal regenerative abilities both in vitro and in vivo. Our findings demonstrate that a TMEM219-dependent ISC death exacerbates colitis and that TMEM219 blockade reestablishes intestinal self-renewal properties in IBD.
Keywords: Apoptosis; Gastroenterology; Inflammatory bowel disease; Mouse models; Stem cells.
Figures



Comment in
- Promoting mucosal healing by targeting TMEM219-dependent intestinal epithelial stem cell defects in inflammatory bowel disease doi: 10.1172/JCI192640

