Anaerobutyricum soehngenii improves glycemic control and other markers of cardio-metabolic health in adults at risk of type 2 diabetes
- PMID: 40371708
- PMCID: PMC12087665
- DOI: 10.1080/19490976.2025.2504115
Anaerobutyricum soehngenii improves glycemic control and other markers of cardio-metabolic health in adults at risk of type 2 diabetes
Abstract
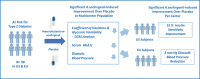
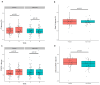
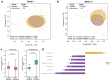
Anaerobutyricum soehngenii (previously Eubacterium hallii) is a butyrate-producing next-generation beneficial microbe generally recognized as safe. Several short-term intervention trials by A. soehngenii L2-7 have shown improvement of insulin sensitivity in prediabetic subjects and type 2 diabetes patients. To determine the long-term cardiometabolic benefits and safety, we performed a 3-month double-blind, randomized placebo-controlled intervention in 98 prediabetic insulin-resistant adults in Europe and U.S. with daily administration of encapsulated cells of A. soehngenii CH-106, a tetracycline-sensitive isogenic derivative of strain L2-7. Compared to placebo, A. soehngenii-treated subjects showed significantly reduced glycemic variability (1% reduction in the coefficient of variation; p = 0.01) and improved glycemic control (6% reduction in the overall net glycemic action-1; p < 0.05), including reduced serum glycated hemoglobin (HbA1c) levels when including the 4-week washout period (1 mmol/mol reduction; p < 0.05). Moreover, diastolic blood pressure was significantly reduced in all A. soehngenii-treated subjects (3 mm Hg; p < 0.05). The study product was well-tolerated and had no effect on the global intestinal microbiota composition, including alpha and beta-diversity, besides an increased abundance of A. soehngenii in the treatment group, indicative of compliance. The U.S. participants, compared to those in Europe, responded best, notably in the oral glucose tolerance tests (15% improvement in the area-under-the curve of plasma glucose levels; p = 0.039) or coefficient of variation (reduction of 3.1%; p < 0.05). This potentially relates to a more severe prediabetic state in U.S. subjects, associated with significantly reduced (1.5-3.5-fold) relative abundance of Bifidobacterium, Coprococcus, Ruminococcus spp. and two-fold increased relative abundance of Lachnoclostridium spp. In conclusion, daily oral supplementation with A. soehngenii was safe and improved various markers of glycemic control, reduced HbA1c levels and diastolic blood pressure, indicating a novel microbiome-based approach to improve cardio-metabolic health in adults at risk for developing type 2 diabetes.Clinical trial reg. no. NCT04529473, clinicaltrials.govSocial media summary 120 characters: Anaerobutyricum soehngenii supplementation improves #cardio-metabolic health in subjects at risk for type 2 #diabetes.
Keywords: Anaerobutyricum; butyrate-producing bacteria; continuous glucose measurement; glycemic control; intestinal microbiota; next-gen beneficial microbes; prediabetes; responder analysis; type 2 diabetes.
Conflict of interest statement
J.K.B., S.H., S.G, J.F.M.L.S, N.B., S.M., and W.M.dV. were employed or received funds from Caelus Health. M.N. received SAB fees which were paid to Amsterdam UMC. M.N. and W.M.dV. are founders and SAB members of Caelus Health.
Figures



References
-
- Sun J, Hu W, Ye S, Deng D, Chen M.. The description and prediction of incidence, prevalence, mortality, disability-adjusted life years cases, and corresponding age-standardized rates for global diabetes. J Epidemiol Glob Health. 2023;13(3):566–20. doi: 10.1007/s44197-023-00138-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
