Transcutaneous Imiquimod Combined With Anti-Programmed Cell Death-1 Monoclonal Antibody Extends the Survival of Mice Bearing Renal Cell Carcinoma
- PMID: 40371846
- PMCID: PMC12079644
- DOI: 10.1002/cam4.70966
Transcutaneous Imiquimod Combined With Anti-Programmed Cell Death-1 Monoclonal Antibody Extends the Survival of Mice Bearing Renal Cell Carcinoma
Abstract
Purpose: Imiquimod (IQM), an imidazoquinoline derivative, is an immunomodulator that activates an adaptive immune response. IQM is applied topically for genital warts and actinic keratosis. Programmed cell death-1 (PD-1) suppresses activated T cells by binding to programmed cell death-ligand 1 and programmed cell death-ligand 2, braking antitumor immunity. Anti-PD-1 therapy has been used for various malignant neoplasms including renal cell carcinoma (RCC). Whether combination therapy with transcutaneous administration of IQM cream and intraperitoneal administration of anti-PD-1 monoclonal antibody (mAb) suppresses mouse RCC cells growing in subcutaneous tissue was investigated.
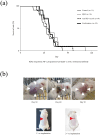
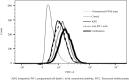
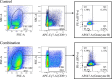
Methods: Female BALB/c mice were implanted subcutaneously with 2 × 105 RENCA mouse RCC cells and treated with a transcutaneously applied cream containing IQM and intraperitoneal administration of anti-PD-1 mAb beginning 5 days after cell implantation. Tumor burden and survival of the mice were determined. RENCA tumor-specific IgG production and a minor CD8+ T cell subset derived from the spleen of the mice bearing RENCA tumor were detected by flow cytometry. The tumor and spleen weights of mice treated with IQM, anti-PD-1 mAb, and their combination were compared.
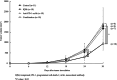
Results: Combination therapy with IQM and anti-PD-1 mAb significantly suppressed tumor growth compared to each monotherapy and prolonged the survival of the mice. The combination therapy produced more RENCA tumor-specific IgG than either IQM or anti-PD-1 mAb alone. The percentage of the CD44highCD62Llow CD8+ T cell subset (effector memory T cells) among splenocytes from mice treated with IQM therapy increased. The CD44lowCD62Llow CD8+ T cell subset (pre-effector-like T cells) of mice treated with anti-PD-1 mAb increased. A negative correlation between tumor and spleen weights was suggested in mice treated with therapies containing IQM.
Conclusions: The present results show that combination therapy with IQM and anti-PD-1 mAb might be a promising novel therapeutic strategy for advanced RCC.
Keywords: IgG; T cell; imiquimod; immune checkpoint inhibitor; programmed cell death‐1; renal cell carcinoma; spleen.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Additional declarations for articles in life science journals that report the results of studies involving humans and/or animals: The care and use of animals in this study were described in a protocol approved by the Kochi Medical School Animal Care and Use Committee; the protocol conformed to Japanese guidelines on the ethical use of animals.
The authors declare no conflicts of interest.
Figures


Similar articles
-
Th2-like response and antitumor effect of anti-interleukin-4 mAb in mice bearing renal cell carcinoma.Cancer Immunol Immunother. 1997 Jan;43(6):375-81. doi: 10.1007/s002620050347. Cancer Immunol Immunother. 1997. PMID: 9067410 Free PMC article.
-
T-cell responses and combined immunotherapy against human carbonic anhydrase 9-expressing mouse renal cell carcinoma.Cancer Immunol Immunother. 2022 Feb;71(2):339-352. doi: 10.1007/s00262-021-02992-7. Epub 2021 Jun 23. Cancer Immunol Immunother. 2022. PMID: 34160685 Free PMC article.
-
Anti-PD-L1 treatment enhances antitumor effect of everolimus in a mouse model of renal cell carcinoma.Cancer Sci. 2016 Dec;107(12):1736-1744. doi: 10.1111/cas.13099. Epub 2016 Dec 13. Cancer Sci. 2016. PMID: 27712020 Free PMC article.
-
PD-1/PD-L1 inhibitors-based treatment for advanced renal cell carcinoma: Mechanisms affecting efficacy and combination therapies.Cancer Med. 2021 Sep;10(18):6384-6401. doi: 10.1002/cam4.4190. Epub 2021 Aug 12. Cancer Med. 2021. PMID: 34382349 Free PMC article. Review.
-
Immune Checkpoint Therapy in Renal Cell Carcinoma.Cancer J. 2016 Mar-Apr;22(2):92-5. doi: 10.1097/PPO.0000000000000177. Cancer J. 2016. PMID: 27111903 Review.
References
-
- Kane C. J., Mallin K., Ritchey J., Cooperberg M. R., and Carroll P. R., “Renal Cell Cancer Stage Migration: Analysis of the National Cancer Data Base,” Cancer 113, no. 1 (2008): 78–83. - PubMed
-
- Sauder D. N., Skinner R. B., Fox T. L., and Owens M. L., “Topical Imiquimod 5% Cream as an Effective Treatment for External Genital and Perianal Warts in Different Patient Populations,” Sexually Transmitted Diseases 30 (2003): 124–128. - PubMed
-
- Hemmi H., Kaisho T., Takeuchi O., et al., “Small Anti‐Viral Compounds Activate Immune Cells via the TLR7 MyD88‐Dependent Signaling Pathway,” Nature Immunology 3, no. 2 (2002): 196–200. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

