Clinical benefit of tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma: real-world data from the EarlyMIND study
- PMID: 40371897
- PMCID: PMC12485315
- DOI: 10.3324/haematol.2024.287141
Clinical benefit of tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma: real-world data from the EarlyMIND study
Abstract
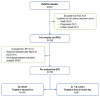
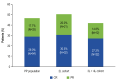
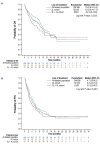
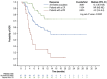
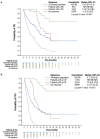
Tafasitamab combined with lenalidomide was approved in Europe in 2021 for transplant-ineligible patients with relapsed/ refractory diffuse large B-cell lymphoma. Approval was based on the L-MIND study, which demonstrated a 57.5% overall response rate (ORR), 41.3% complete response (CR) rate, 12.1-month median progression-free survival (PFS), and 33.5-month median overall survival (OS) at 5 years. The multicenter retrospective EarlyMIND study analyzed real-world efficacy and treatment patterns of patients receiving tafasitamab plus lenalidomide for second-line (2L cohort) to fourth-line (3L + 4L cohort) treatment in the French Early Access Program. Outcomes were analyzed overall and according to number of previous lines of treatment. Post hoc analyses were conducted on subgroups based on prognostic factors, performance status, primary refractoriness, cell of origin, and treatment response. Overall, 186 patients were included (2L: N=105; 3L + 4L: N=81). The median age of the patients was 78 years; most patients had early relapsed disease (71.2%), including 60.2% with primary refractory disease. At a median follow-up of 8.2 months, best ORR was 46.8%, with a CR rate of 29%. The ORR was higher in the 2L cohort (50.5%) than in the 3L + 4L cohort (42%). The median PFS and OS were 4.7 and 10 months, respectively, in the overall population, 5.4 and 10.6 months in the 2L cohort, and 3.6 and 8.2 months in the 3L + 4L cohort. Long-lasting responses were observed in patients achieving CR, with median duration of response, PFS, and OS not reached. The median time to CR as best objective response was four cycles, regardless of treatment line. Despite involving a frail population with high-risk disease characteristics, results of this large real-world European retrospective study on tafasitamab plus lenalidomide are encouraging, with almost one third of patients experiencing long-lasting CR.
Figures
References
-
- Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5: v116-v125. - PubMed
-
- Kanas G, Ge W, Quek RGW, Keeven K, Nersesyan K, Arnason JE. Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: population-level projections for 2020-2025. Leuk Lymphoma. 2022;63(1):54-63. - PubMed
-
- Defossez G, Le Guyader-Peyrou S, Uhry Z, et al. National estimates of cancer incidence and mortality in metropolitan France between 1990 and 2018 - Volume 1: solid tumors: study based on the cancer registries of the Francim network. 09/2019 [Article in French]. https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/canc... Accessed October 7, 2024.
-
- Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology. 2018;50(1):74-87. - PubMed
-
- Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12(5):460-468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

