AARS2 ameliorates myocardial ischemia via fine-tuning PKM2-mediated metabolism
- PMID: 40371904
- PMCID: PMC12080999
- DOI: 10.7554/eLife.99670
AARS2 ameliorates myocardial ischemia via fine-tuning PKM2-mediated metabolism
Abstract
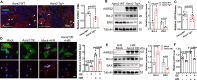
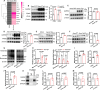
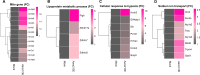
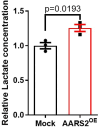
AARS2, an alanyl-tRNA synthase, is essential for protein translation, but its function in mouse hearts is not fully addressed. Here, we found that cardiomyocyte-specific deletion of mouse AARS2 exhibited evident cardiomyopathy with impaired cardiac function, notable cardiac fibrosis, and cardiomyocyte apoptosis. Cardiomyocyte-specific AARS2 overexpression in mice improved cardiac function and reduced cardiac fibrosis after myocardial infarction (MI), without affecting cardiomyocyte proliferation and coronary angiogenesis. Mechanistically, AARS2 overexpression suppressed cardiomyocyte apoptosis and mitochondrial reactive oxide species production, and changed cellular metabolism from oxidative phosphorylation toward glycolysis in cardiomyocytes, thus leading to cardiomyocyte survival from ischemia and hypoxia stress. Ribo-Seq revealed that Aars2 overexpression increased pyruvate kinase M2 (PKM2) protein translation and the ratio of PKM2 dimers to tetramers that promote glycolysis. Additionally, PKM2 activator TEPP-46 reversed cardiomyocyte apoptosis and cardiac fibrosis caused by AARS2 deficiency. Thus, this study demonstrates that AARS2 plays an essential role in protecting cardiomyocytes from ischemic pressure via fine-tuning PKM2-mediated energy metabolism, and presents a novel cardiac protective AARS2-PKM2 signaling during the pathogenesis of MI.
Keywords: AARS2; PKM2; cardiac remodeling; cardiomyocytes; cell biology; glycolysis; medicine; mouse.
© 2024, Zhang et al.
Conflict of interest statement
ZZ, LZ, YC, YC, JH, CX, XZ, SZ, JX No competing interests declared
Figures














Update of
- doi: 10.1101/2024.06.04.597368
- doi: 10.7554/eLife.99670.1
- doi: 10.7554/eLife.99670.2
References
-
- Dallabona C, Diodato D, Kevelam SH, Haack TB, Wong L-J, Salomons GS, Baruffini E, Melchionda L, Mariotti C, Strom TM, Meitinger T, Prokisch H, Chapman K, Colley A, Rocha H, Ounap K, Schiffmann R, Salsano E, Savoiardo M, Hamilton EM, Abbink TEM, Wolf NI, Ferrero I, Lamperti C, Zeviani M, Vanderver A, Ghezzi D, van der Knaap MS. Novel (ovario) leukodystrophy related to AARS2 mutations. Neurology. 2014;82:2063–2071. doi: 10.1212/WNL.0000000000000497. - DOI - PMC - PubMed
-
- Dayton TL, Gocheva V, Miller KM, Israelsen WJ, Bhutkar A, Clish CB, Davidson SM, Luengo A, Bronson RT, Jacks T, Vander Heiden MG. Germline loss of PKM2 promotes metabolic distress and hepatocellular carcinoma. Genes & Development. 2016a;30:1020–1033. doi: 10.1101/gad.278549.116. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- SQ2023YFA1800026/National Key Research and Development Program of China
- 2018YFA0800501/National Key Research and Development Program of China
- 32230032/National Natural Science Foundation of China
- 31730061/National Natural Science Foundation of China
- 81870198/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

