Pools of Independently Cycling Inositol Phosphates Revealed by Pulse Labeling with 18O-Water
- PMID: 40372010
- PMCID: PMC12123611
- DOI: 10.1021/jacs.4c16206
Pools of Independently Cycling Inositol Phosphates Revealed by Pulse Labeling with 18O-Water
Abstract
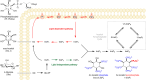
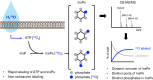
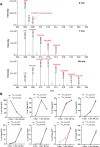
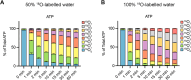
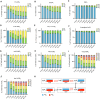
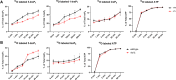
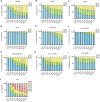
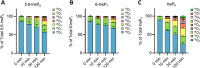
Inositol phosphates control many central processes in eukaryotic cells including nutrient availability, growth, and motility. Kinetic resolution of a key modulator of their signaling functions, the turnover of the phosphate groups on the inositol ring, has been hampered by slow uptake, high dilution, and constraining growth conditions in radioactive pulse-labeling approaches. Here, we demonstrate a rapid (seconds to minutes) and nonradioactive labeling strategy of inositol polyphosphates through 18O-water in yeast, human cells, and amoeba, which can be applied in any media. In combination with capillary electrophoresis and mass spectrometry, 18O-water labeling simultaneously dissects the in vivo phosphate group dynamics of a broad spectrum of even rare inositol phosphates. The good temporal resolution allowed us to discover vigorous phosphate group exchanges in some inositol polyphosphates and pyrophosphates, whereas others remain remarkably inert. We propose a model in which the biosynthetic pathway of inositol polyphosphates and pyrophosphates is organized in distinct, kinetically separated pools. While transfer of compounds between those pools is slow, each pool undergoes rapid internal phosphate cycling. This might enable the pools to perform distinct signaling functions while being metabolically connected.
Figures
Similar articles
-
Stable Isotope Phosphate Labelling of Diverse Metabolites is Enabled by a Family of 18 O-Phosphoramidites.Angew Chem Int Ed Engl. 2022 Jan 26;61(5):e202112457. doi: 10.1002/anie.202112457. Epub 2021 Nov 23. Angew Chem Int Ed Engl. 2022. PMID: 34734451 Free PMC article.
-
Extraction and analysis of soluble inositol polyphosphates from yeast.Nat Protoc. 2006;1(5):2416-22. doi: 10.1038/nprot.2006.337. Nat Protoc. 2006. PMID: 17406485
-
Phosphate, inositol and polyphosphates.Biochem Soc Trans. 2016 Feb;44(1):253-9. doi: 10.1042/BST20150215. Biochem Soc Trans. 2016. PMID: 26862212 Review.
-
Analysis of inositol phosphate metabolism by capillary electrophoresis electrospray ionization mass spectrometry.Nat Commun. 2020 Nov 27;11(1):6035. doi: 10.1038/s41467-020-19928-x. Nat Commun. 2020. PMID: 33247133 Free PMC article.
-
Importance of Radioactive Labelling to Elucidate Inositol Polyphosphate Signalling.Top Curr Chem (Cham). 2017 Feb;375(1):14. doi: 10.1007/s41061-016-0099-y. Epub 2017 Jan 18. Top Curr Chem (Cham). 2017. PMID: 28101851 Free PMC article. Review.
Cited by
-
Synthesis of monodisperse inorganic polyphosphate polyP10 via a photocaging strategy.Chem Sci. 2025 Jul 10. doi: 10.1039/d5sc04037j. Online ahead of print. Chem Sci. 2025. PMID: 40666202 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

