Age-associated changes in the heart: implications for COVID-19 therapies
- PMID: 40372276
- PMCID: PMC12151517
- DOI: 10.18632/aging.206251
Age-associated changes in the heart: implications for COVID-19 therapies
Abstract
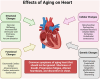
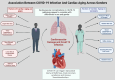
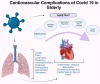
Cardiac aging involves progressive structural, functional, cellular, and molecular changes that impair heart function. This review explores key mechanisms, including oxidative stress, mitochondrial dysfunction, impaired autophagy, and chronic low-grade inflammation. Excess reactive oxygen species (ROS) damage heart muscle cells, contributing to fibrosis and cellular aging. Mitochondrial dysfunction reduces energy production and increases oxidative stress, accelerating cardiac decline. Impaired autophagy limits the removal of damaged proteins and organelles, while inflammation activates signaling molecules that drive tissue remodeling. Gender differences reveal estrogen's protective role in premenopausal women, with men showing greater susceptibility to heart muscle dysfunction and injury. After menopause, women lose this hormonal protection, increasing their risk of cardiovascular conditions. Ethnic disparities, particularly among underserved minority populations, emphasize how social factors such as access to care, environment, and chronic stress contribute to worsening cardiovascular outcomes. The coronavirus disease pandemic has introduced further challenges by increasing the incidence of heart damage through inflammation, blood clots, and long-term heart failure, especially in older adults with existing metabolic conditions like diabetes and high blood pressure. The virus's interaction with receptors on heart and blood vessel cells, along with a weakened immune response in older adults, intensifies cardiac aging. Emerging therapies include delivery of therapeutic extracellular vesicles, immune cell modulation, and treatments targeting mitochondria. In addition, lifestyle strategies such as regular physical activity, nutritional improvements, and stress reduction remain vital to maintaining cardiac health. Understanding how these biological and social factors intersect is critical to developing targeted strategies that promote healthy aging of the heart.
Keywords: COVID-19 cardiovascular complications; cardiac aging; health disparities; mitochondrial dysfunction; oxidative stress.
Conflict of interest statement
Figures



Similar articles
-
Systemic aging fuels heart failure: Molecular mechanisms and therapeutic avenues.ESC Heart Fail. 2025 Apr;12(2):1059-1080. doi: 10.1002/ehf2.14947. Epub 2024 Jul 22. ESC Heart Fail. 2025. PMID: 39034866 Free PMC article. Review.
-
Metabolic Syndrome and Cardiac Remodeling Due to Mitochondrial Oxidative Stress Involving Gliflozins and Sirtuins.Curr Hypertens Rep. 2023 Jun;25(6):91-106. doi: 10.1007/s11906-023-01240-w. Epub 2023 Apr 13. Curr Hypertens Rep. 2023. PMID: 37052810 Review.
-
Vesicoureteral Reflux.2024 Apr 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Apr 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 33085409 Free Books & Documents.
-
New insights into the role of mitochondrial dynamics and autophagy during oxidative stress and aging in the heart.Oxid Med Cell Longev. 2014;2014:210934. doi: 10.1155/2014/210934. Epub 2014 Jul 15. Oxid Med Cell Longev. 2014. PMID: 25132912 Free PMC article. Review.
-
Staying young at heart: autophagy and adaptation to cardiac aging.J Mol Cell Cardiol. 2016 Jun;95:78-85. doi: 10.1016/j.yjmcc.2015.11.006. Epub 2015 Nov 5. J Mol Cell Cardiol. 2016. PMID: 26549356 Free PMC article. Review.
References
-
- Marín-Aguilar F, Lechuga-Vieco AV, Alcocer-Gómez E, Castejón-Vega B, Lucas J, Garrido C, Peralta-Garcia A, Pérez-Pulido AJ, Varela-López A, Quiles JL, Ryffel B, Flores I, Bullón P, et al. NLRP3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging Cell. 2020; 19:e13050. 10.1111/acel.13050 - DOI - PMC - PubMed
-
- Nakamura K, Miyoshi T, Yoshida M, Akagi S, Saito Y, Ejiri K, Matsuo N, Ichikawa K, Iwasaki K, Naito T, Namba Y, Yoshida M, Sugiyama H, Ito H. Pathophysiology and Treatment of Diabetic Cardiomyopathy and Heart Failure in Patients with Diabetes Mellitus. Int J Mol Sci. 2022; 23:3587. 10.3390/ijms23073587 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

