Mast cells-intestinal cancer cells crosstalk is mediated by TNF-alpha and sustained by the IL-33/ST2 axis
- PMID: 40372523
- PMCID: PMC12081814
- DOI: 10.1007/s00262-025-04054-8
Mast cells-intestinal cancer cells crosstalk is mediated by TNF-alpha and sustained by the IL-33/ST2 axis
Abstract
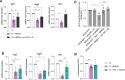
It is common knowledge that mast cells (MCs) exert different roles in the gastrointestinal tract, from the maintenance of homeostasis to the onset and propagation of different gut diseases such as food allergies, infections, inflammation, and cancer. However, the mechanisms through which MCs dialog and influence the intestinal tissue are not completely known. To get insight into the bidirectional crosstalk between MCs and the intestinal microenvironment, both in homeostatic and pathological settings, colon organoids from intestinal epithelium of healthy mice and adenomas from AOM/DSS-treated mice have been exploited and co-cultured with MCs. The influence of MCs on organoid architecture and the effect of healthy and tumoral organoids on the phenotype and responsiveness of MCs have been addressed. We observed that MCs interact with intestinal organoids and contribute to the differentiation of healthy organoids by upregulating the expression of mucin-2, chromogranin A, cadherin-1, and claudin 4. On the contrary, in co-culture with tumoral organoids a decrease in cell proliferation, chromogranin A, and lysozyme expression was observed. Tumoral organoids have been shown to activate MCs via the IL-33/ST2 axis leading to increased release of TNF-α which in turn was responsible for the observed effects on tumoral organoids. Our results indicate that MCs are important mediators of intestinal tissue homeostasis and that a different environment can shape and direct MCs toward the dampening or propagation of the inflammatory response. Ultimately, our MC-organoid co-cultures represent a valid in vitro tool to investigate the role of MCs in the gut.
Keywords: Co-cultures; IL-33; Intestinal differentiation and architecture; Mast cells; Organoids; TNF-α.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests.
Figures




References
-
- Bischoff SC, Krämer S (2007) Human mast cells, bacteria, and intestinal immunity. Immunol Rev 217:329–337. 10.1111/J.1600-065X.2007.00523.X - PubMed
-
- Bischoff SC (2009) Physiological and pathophysiological functions of intestinal mast cells. Semin Immunopathol 31:185–205. 10.1007/s00281-009-0165-4 - PubMed
-
- Merluzzi S, Frossi B, Gri G et al (2010) Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood 115:2810–2817. 10.1182/blood-2009-10-250126 - PubMed
MeSH terms
Substances
Grants and funding
- Interdepartmental Project on Healthy Ageing (2020-25)./Università degli Studi di Udine
- Interdepartmental Project on Healthy Ageing (2020-25)./Università degli Studi di Udine
- Interdepartmental Project on Healthy Ageing (2020-25)./Università degli Studi di Udine
- Interdepartmental Project on Healthy Ageing (2020-25)./Università degli Studi di Udine
- AIRC_IG_2022_ID_27884_PUCILLO/Fondazione AIRC per la ricerca sul cancro ETS
LinkOut - more resources
Full Text Sources
Research Materials

