Peripheral Neuropathy Symptoms and Ocular Surface Lesions in Patients with Type 2 Diabetes Mellitus and Dry Eye: A Clinical Correlational Study
- PMID: 40372617
- PMCID: PMC12167187
- DOI: 10.1007/s40123-025-01150-x
Peripheral Neuropathy Symptoms and Ocular Surface Lesions in Patients with Type 2 Diabetes Mellitus and Dry Eye: A Clinical Correlational Study
Abstract
Introduction: Reduced corneal sensation in individuals with type 2 diabetes mellitus (T2DM) leads to a dissociation between dry eye disease (DED) signs and symptoms, thereby affecting diagnostic accuracy. This study aimed to investigate the correlation between ocular surface signs and diabetic peripheral neuropathy (DPN) symptoms in patients with T2DM-associated DED.
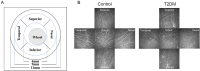
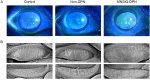
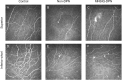
Methods: The Michigan Neuropathy Screening Instrument Questionnaire (MNSIQ) was used to categorize patients with T2DM into MNSIQ-DPN and non-DPN groups. Ocular irritation symptoms were evaluated using the Ocular Surface Disease Index (OSDI) questionnaire. Ocular surface lesions were assessed via Cochet-Bonnet esthesiometry, corneal fluorescein staining (CFS), the Schirmer I tear test (SIT), tear meniscus height (TMH), noninvasive keratography break-up time (NIKf-BUT), and the meibomian gland loss (MGL) grade detected by OCULUS. Corneal nerve fiber parameters were evaluated using in vivo confocal microscopy (IVCM).
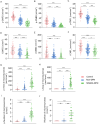
Results: A total of 116 patients with T2DM, comprising 76 non-DPN patients and 40 MNSIQ-DPN patients, along with 51 age-matched participants without diabetes, were enrolled. Although OSDI scores were equivalent between MNSIQ-DPN patients and non-DPN patients, MNSIQ-DPN patients presented significantly more severe CFS (p < 0.001), meibomian gland dysfunction (MGD) (p < 0.001), corneal nerve fiber loss (p < 0.001), sensory dysfunction (p = 0.02), and corneal microneuromas (p < 0.001). The MNSIQ score was significantly positively correlated with CFS (p < 0.001); MGD (p < 0.01); corneal nerve fiber loss, including corneal nerve fiber density and length and branch density, in the paracentral (all p < 0.001) and inferior-whorl areas (p < 0.01, p < 0.05 and p < 0.01, respectively); and corneal microneuromas, characterized by increased microneuroma numbers (p < 0.001) and areas (p < 0.001) in these regions.
Conclusion: MNSIQ scores were significantly and robustly correlated with the presence of corneal epithelial defects, MGD, and nerve fiber loss in patients with T2DM. These findings suggest that DPN is a critical factor in diabetic ocular surface complications, highlighting the importance of the MNSIQ for assessing these conditions.
Keywords: Diabetes; Diabetic peripheral neuropathy; Dry eye disease; In vivo confocal microscopy; Meibomian gland dysfunction; Microneuroma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Yanling Liu, Dapeng Sun, Qianqian Kong, Dongfang Li, Rui Wang, Jia Yin, Lixin Xie, Yanling Dong, and Yangyang Zhang declare that they have no conflicts of interest. The authors alone are responsible for the content and writing of the paper. Ethical Approval: The study protocol was approved by the local Committee of Qingdao Eye Hospital Research Ethics (approval number: 2019-33), and the procedures were performed in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants or their legal guardians.
Figures
Similar articles
-
Correlation of the retinopathy degree with the change of ocular surface and corneal nerve in patients with type 2 diabetes mellitus.Int J Ophthalmol. 2021 May 18;14(5):750-758. doi: 10.18240/ijo.2021.05.17. eCollection 2021. Int J Ophthalmol. 2021. PMID: 34012892 Free PMC article.
-
Association of peripheral neuropathy with dry eye disease and meibomian gland dysfunction in patients with type 1 diabetes.Int Ophthalmol. 2025 Feb 7;45(1):61. doi: 10.1007/s10792-025-03420-w. Int Ophthalmol. 2025. PMID: 39920472
-
Influence of video display terminal use and meibomian gland dysfunction on the ocular surface and tear neuromediators.Int Ophthalmol. 2023 May;43(5):1537-1544. doi: 10.1007/s10792-022-02549-2. Epub 2022 Oct 14. Int Ophthalmol. 2023. PMID: 36239837
-
Corneal microstructural changes, and nephropathy, in participants with diabetes mellitus with and without peripheral neuropathy: a systematic review and meta-analysis.Clin Exp Optom. 2025 May 18:1-12. doi: 10.1080/08164622.2025.2499601. Online ahead of print. Clin Exp Optom. 2025. PMID: 40383529 Review.
-
Prospective evaluation of a new intense pulsed light, thermaeye plus, in the treatment of dry eye disease due to meibomian gland dysfunction.J Optom. 2021 Apr-Jun;14(2):103-113. doi: 10.1016/j.optom.2020.08.009. Epub 2020 Oct 26. J Optom. 2021. PMID: 33121905 Free PMC article. Review.
Cited by
-
Relationship between type 2 diabetes mellitus and changes of the lid margin, meibomian gland and tear film in dry eye patients: a critical perspective.Int Ophthalmol. 2025 Aug 23;45(1):347. doi: 10.1007/s10792-025-03739-4. Int Ophthalmol. 2025. PMID: 40848076 No abstract available.
References
-
- Neeland IJ, Patel KV. Diabetes. In: Biomarkers in cardiovascular disease. 2019. p. 41–51.
LinkOut - more resources
Full Text Sources

