Autoimmune bullous diseases: pathogenesis and clinical management
- PMID: 40372624
- PMCID: PMC12081819
- DOI: 10.1186/s43556-025-00272-9
Autoimmune bullous diseases: pathogenesis and clinical management
Abstract
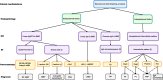
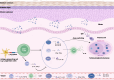
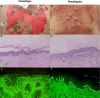
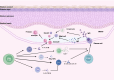
Autoimmune bullous diseases (AIBDs) represent a heterogeneous group of immune-mediated disorders characterized by life-threatening blistering of the skin and mucous membranes. This Review synthesizes current understanding of AIBD pathogenesis, clinical phenotypes, diagnostic approaches, and therapeutic strategies, emphasizing recent advancements and translational opportunities. At the core of AIBDs is autoantibody-mediated disruption of structural proteins in the epidermis or basement membrane zone, particularly at desmosomal and hemidesmosomal junctions. Key subtypes, including pemphigus, paraneoplastic pemphigus, pemphigoid, and IgA-related diseases, are distinguished by their target antigens, clinical manifestations, and immunopathological profiles. Diagnostic workflows rely on direct immunofluorescence, and serological assays, yet subtype differentiation remains challenging due to overlapping features. Traditional therapies, such as systemic corticosteroids and immunosuppressants, have improved outcomes but are limited by toxicity. Recent breakthroughs highlight targeted interventions, including B-cell depletion with rituximab, cytokine modulation via dupilumab, and JAK inhibitors for inflammatory pathways. Innovative strategies like chimeric autoantibody receptor T-cell (CAART) therapy further address refractory cases by eliminating autoreactive B cells. Additionally, the Review underscores the emerging role of inflammation-driven mechanisms and the necessity of multidisciplinary care, given AIBDs' associations with malignancies, autoimmune comorbidities. Despite progress, challenges persist in early diagnosis, personalized therapy optimization, and understanding antigen-specific immune responses. Future directions include refining diagnostic biomarkers, exploring novel targets, and developing precision medicine approaches.
Keywords: Autoantibody; Autoimmune bullous diseases; Immunogenic domain; Inflammation; Pathogenesis; Targeted therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All participants in this study were derived from the autoimmune bullous disease cohort of West China Hospital (AIBD-WCH). The AIBD-WCH was established in 2016, which was approved by the biomedical research ethics committee of West China Hospital of Sichuan University (Approval number: 2017–241). Written informed consent was obtained from all participants. Competing interests: The authors have no relevant financial or non-financial interests to disclose.
Figures
Similar articles
-
Regulatory T-cell deficiency leads to pathogenic bullous pemphigoid antigen 230 autoantibody and autoimmune bullous disease.J Allergy Clin Immunol. 2018 Dec;142(6):1831-1842.e7. doi: 10.1016/j.jaci.2018.04.006. Epub 2018 Apr 26. J Allergy Clin Immunol. 2018. PMID: 29704595
-
Classification and Antigen Molecules of Autoimmune Bullous Diseases.Biomolecules. 2023 Apr 20;13(4):703. doi: 10.3390/biom13040703. Biomolecules. 2023. PMID: 37189450 Free PMC article. Review.
-
Diagnosis of autoimmune bullous diseases.J Dtsch Dermatol Ges. 2018 Sep;16(9):1077-1091. doi: 10.1111/ddg.13637. J Dtsch Dermatol Ges. 2018. PMID: 30179336 Review.
-
Retrospective analysis of autoimmune bullous diseases in Middle Franconia.Front Immunol. 2023 Oct 10;14:1256617. doi: 10.3389/fimmu.2023.1256617. eCollection 2023. Front Immunol. 2023. PMID: 37881435 Free PMC article.
-
[Modern diagnostics of autoimmune bullous diseases].Pathologe. 2020 Jul;41(4):317-325. doi: 10.1007/s00292-020-00795-8. Pathologe. 2020. PMID: 32542511 Review. German.
References
-
- Bystryn JC, Rudolph JL. Pemphigus. Lancet. 2005;366(9479):61–73. 10.1016/S0140-6736(05)66829-8. - PubMed
-
- Schmidt E, Zillikens D. Pemphigoid diseases. Lancet. 2013;381(9863):320–32. 10.1016/S0140-6736(12)61140-4. - PubMed
-
- King DF, Holubar K. History of pemphigus. Clin Dermatol. 1983;1(2):6–12. 10.1016/0738-081x(83)90019-6. - PubMed
-
- Lever WF. Pemphigus. Medicine (Baltimore). 1953;32(1):1–23. 10.1097/00005792-195302000-00001. - PubMed
-
- Beutner EH, Jordon RE. Demonstration of skin antibodies in sera of pemphigus vulgaris patients by indirect immunofluorescent staining. Proc Soc Exp Biol Med. 1964;117:505–10. 10.3181/00379727-117-29622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82304062/the National Natural Science Foundation of China
- 2024NSFSC1626/the Natural Science Foundation of Sichuan Province
- 2023SCU12065/the Postdoctoral Fund of Sichuan University
- 2024HXBH182/Innovative Research Group Project of the National Natural Science Foundation of China
- 2024M752267/Sichuan Provincial Postdoctoral Science Foundation
- 2023-YF09-00003-SN/the Achievement Transformation Demonstration Project of the Key research and development (R&D) Program of Science & Technology Department of Chengdu
- 2023HXBH098/the Postdoctoral Research Fund of West China Hospital, Sichuan University
- 2023NSFSC1551/Natural Science Foundation of Sichuan Province
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
