Use of Biologics During Pregnancy Among Patients With Autoimmune Conditions
- PMID: 40372753
- PMCID: PMC12082371
- DOI: 10.1001/jamanetworkopen.2025.10504
Use of Biologics During Pregnancy Among Patients With Autoimmune Conditions
Abstract
Importance: Continuation of biologics in patients with an autoimmune condition who become pregnant involves weighing consequences of pregnancy-related changes in disease severity and potential teratogenic effects of medications. Characterization of biologic treatment patterns during pregnancy may provide insight into maternal and fetal risks and benefits.
Objective: To describe the utilization pattern of biologics in pregnant individuals with autoimmune conditions.
Design, setting, and participants: This cohort study used data from Merative MarketScan Research Databases, which contain administrative claims of commercially insured individuals in the US. Pregnant patients aged 16 to 55 years with an autoimmune condition and biologic use 6 months before conception between January 1, 2011, and December 31, 2022, were included. The data were analyzed between October 15, 2024, and February 28, 2025.
Exposure: Use of biologics for autoimmune disease after conception.
Main outcomes and measures: The proportion of patients who used biologics for Crohn disease, ulcerative colitis, psoriasis or psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus, and multiple sclerosis was assessed, and the association between underlying autoimmune disease and use of biologics during pregnancy was measured using multivariable logistic regression.
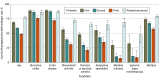
Results: A total of 6131 pregnant patients (median [IQR] age, 32 [29-36] years) with an autoimmune condition were included. The most prevalent conditions were Crohn disease (1372 patients [25.6%]) and rheumatoid arthritis (1295 patients [24.1%]). Of all patients, 4393 (71.6%; 95% CI, 70.5%-72.8%) used biologics at least once during pregnancy. Among pregnancies with live birth outcomes, biologic use declined throughout gestation, with 2981 patients (68.6% [95% CI, 67.2%-70.0%]), 2555 patients (58.8% [95% CI, 57.3%-60.3%]), and 2113 patients (48.6% [95% CI, 47.1%-50.1%]) using biologics during the first, second, and third trimesters, respectively, and 3350 patients (77.1% [95% CI, 75.8%-78.3%]) using them post partum. Compared with pregnant patients with rheumatoid arthritis, those with Crohn disease (odds ratio [OR], 7.88 [95% CI, 5.93-10.47]) and ulcerative colitis (OR, 5.35 [95% CI, 3.73-7.66]) were more likely to use biologics, while those with psoriasis or psoriatic arthritis (OR, 0.65 [95% CI, 0.52-0.80]) were less likely.
Conclusions and relevance: In this cohort study, a decline in the use of biologics for autoimmune disease was observed during the pregnancy period that rebounded only partially thereafter. Notable variations in use across autoimmune conditions suggest that indication-specific risk-benefit assessments of biologic use are needed.
Conflict of interest statement
Figures


References
-
- Russell MD, Dey M, Flint J, et al. ; BSR Standards, Audit and Guidelines Working Group . British Society for Rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: immunomodulatory anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford). 2023;62(4):e48-e88. doi:10.1093/rheumatology/keac551 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

