Genetic variants predisposing to an increased risk of kidney stone disease
- PMID: 40372791
- PMCID: PMC12321396
- DOI: 10.1172/JCI186915
Genetic variants predisposing to an increased risk of kidney stone disease
Abstract
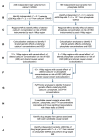
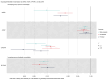
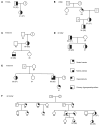
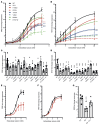
BACKGROUNDKidney stone disease (KSD) affects approximately 10% of adults, is heritable, and is associated with mineral metabolic abnormalities.METHODSGenetic variants and pathways increasing KSD risk via calcium and phosphate homeostasis were ascertained using GWAS, region-specific Mendelian randomization (MR), and genetic colocalization. The utility of pathway modulation was estimated via drug target MR, and the effects of variants on calcium-sensing receptor (CaSR) signaling were characterized.RESULTSSeventy-nine independent KSD-associated genetic signals at 71 loci were identified. MR identified 3 loci affecting KSD risk via increased serum calcium or decreased serum phosphate concentrations (ORs for genomic regions = 4.30, 11.42, and 13.83 per 1 SD alteration; P < 5.6 × 10-10). Colocalization analyses defined putative, noncoding KSD-causing variants estimated to account for 11%-19% of KSD cases in proximity to diacylglycerol kinase δ (DGKD), a CaSR signaling partner; solute carrier family 34 member 1 (SLC34A1), a renal sodium-phosphate transporter; and cytochrome P450 family 24 subfamily A member 1 (CYP24A1), which degrades 1,25-dihydroxyvitamin D. Drug target MR indicated that reducing serum calcium by 0.08 mmol/L via CASR, DGKD, or CYP24A1, or increasing serum phosphate by 0.16 mmol/L via SLC34A1 may reduce KSD relative risk by up to 90%. Furthermore, reduced DGKδ expression and KSD-associated DGKD missense variants impaired CaSR signal transduction in vitro, which was ameliorated by cinacalcet, a positive CaSR allosteric modulator.CONCLUSIONDGKD-, SLC34A1-, and CYP24A1-associated variants linked to reduced CaSR signal transduction, increased urinary phosphate excretion, and impaired 1,25-dihydroxyvitamin D inactivation, respectively, are common causes of KSD. Genotyping patients with KSD may facilitate personalized KSD risk stratification and targeted pharmacomodulation of associated pathways to prevent KSD.FUNDINGOxfordshire Health Services Research Committee (OHSRC, part of Oxford Hospitals Charity); Kidney Research UK (RP_030_20180306); The Urology Foundation; National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (NF-SI-0514-10091); Wellcome Trust (204826/z/16/z and 106995/z/15/z); Medical Research Council (MRC) Clinical Research Training Fellowships (MR/W03168X/1 and MR/S021329/1); Wellcome Trust Clinical Career Development Fellowship; Sir Henry Dale Fellowship, with joint funding by the Wellcome Trust and the Royal Society (224155/Z/21/Z); St. Peter's Trust for Kidney Bladder and Prostate Research.
Keywords: Calcium signaling; Endocrinology; Genetic variation; Genetics; Nephrology; Urology.
Conflict of interest statement
Figures

Comment in
- Genetic susceptibility to kidney stone disease: unveiling pathogenesis and potential therapeutic targets
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

