Live bacteria in gut microbiome dictate asthma onset triggered by environmental particles via modulation of DNA methylation in dendritic cells
- PMID: 40372916
- PMCID: PMC12172552
- DOI: 10.1016/j.celrep.2025.115684
Live bacteria in gut microbiome dictate asthma onset triggered by environmental particles via modulation of DNA methylation in dendritic cells
Abstract
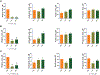
Despite broad knowledge of the pathogenesis, our understanding of the origin of allergy and asthma remains poor, preventing etiotropic treatments. The gut microbiome is seen to be altered in asthmatics; however, proof of causality of the microbiome alterations is lacking. We report on gut microbiome transplantation (GMT) from mice predisposed to asthma by maternal exposure to pro-allergy environmental particles into naive recipients. This GMT confers asthma predisposition, and the effect is abrogated by gamma sterilization of the transplant material or by co-administration of antibacterials, indicating that viable bacteria are mediating the effect. Metagenomics identifies key changes in the "pro-asthma" microbiome, and metabolomics links the identified species to altered production of butyrate known to act on immune cells and epigenetic mechanisms. We further show that transplant recipients develop DNA methylation alterations in dendritic cells. Finally, dendritic cells with an altered methylome present allergen to T cells, and this effect is abrogated by an epigenetically acting drug in vitro.
Keywords: CAP; CP: Immunology; CP: Microbiology; DEP; DNA methylation; GMT; allergy; antibiotics; asthma; concentrated urban air particles; dendritic cells; diesel exhaust particles; epigenetics; gut microbiome transplant; radiation sterilization.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
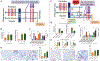
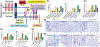




Similar articles
-
Link between epigenomic alterations and genome-wide aberrant transcriptional response to allergen in dendritic cells conveying maternal asthma risk.PLoS One. 2013 Aug 12;8(8):e70387. doi: 10.1371/journal.pone.0070387. eCollection 2013. PLoS One. 2013. PMID: 23950928 Free PMC article.
-
Transgenerational transmission of asthma risk after exposure to environmental particles during pregnancy.Am J Physiol Lung Cell Mol Physiol. 2017 Aug 1;313(2):L395-L405. doi: 10.1152/ajplung.00035.2017. Epub 2017 May 11. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28495853 Free PMC article.
-
Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo.Toxicol Sci. 2008 Mar;102(1):76-81. doi: 10.1093/toxsci/kfm290. Epub 2007 Nov 27. Toxicol Sci. 2008. PMID: 18042818 Free PMC article.
-
Understanding Asthma and Allergies by the Lens of Biodiversity and Epigenetic Changes.Front Immunol. 2021 Mar 1;12:623737. doi: 10.3389/fimmu.2021.623737. eCollection 2021. Front Immunol. 2021. PMID: 33732246 Free PMC article. Review.
-
Environmental epigenetics of asthma: an update.J Allergy Clin Immunol. 2010 Sep;126(3):453-65. doi: 10.1016/j.jaci.2010.07.030. J Allergy Clin Immunol. 2010. PMID: 20816181 Free PMC article. Review.
References
-
- US CDC. Asthma Surveillance in the United States, 2001–2021. Presentation derived from https://www.cdc.gov/asthma/asthma-prevalence-us-2023-508.pdf on November/22/24. Data are derived from https://www.cdc.gov/asthma-data/about/index.html on 11/22/24.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

