Engineering of long-acting human growth hormone-Fc fusion proteins: Effects of valency, fusion position, and linker design on pharmacokinetics and efficacy
- PMID: 40373007
- PMCID: PMC12080763
- DOI: 10.1371/journal.pone.0323791
Engineering of long-acting human growth hormone-Fc fusion proteins: Effects of valency, fusion position, and linker design on pharmacokinetics and efficacy
Abstract
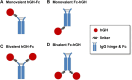
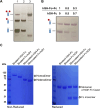
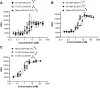
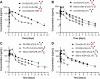
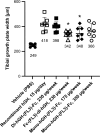
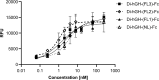
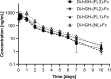
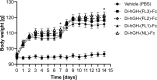
Fc fusion proteins, formed by fusing an active protein to the Fc region of immunoglobulin G, are a validated strategy for extending the half-life of therapeutic proteins. Human growth hormone (hGH) Fc fusion proteins exhibit longer circulation half-lives than hGH, reducing injection frequency and improving convenience for hGH replacement therapy. Most approved Fc fusion proteins involve directly attaching the active protein to the hinge region of IgG Fc; however, few reports have described the effects of structural variations on these characteristics in detail. We analyzed pharmacokinetic and pharmacodynamic properties of various hGH-Fc fusion constructs differing in linker type, hGH valency, and fusion position to investigate the structure-function relationships of these proteins in cell-based assays and animal models, including normal and hypophysectomized rats. Monovalent hGH-Fc fusion variants and those with hGH fused to the C-terminal of IgG Fc exhibited higher in vitro and in vivo activity than bivalent hGH-Fc. However, these variants also exhibited accelerated clearance in rat pharmacokinetic experiments. The linker connecting the hGH moiety to the Fc domain significantly influenced in vitro activity and pharmacokinetics. Constructs with a rigid alpha-helical A(EAAAK)5A linker showed greater in vitro activity than those with a flexible (GGGGS)3 linker but exhibited accelerated clearance in rats. To a lesser extent, linker length influenced activity and pharmacokinetics. Bivalent hGH-Fc constructs with shorter linkers (0-1 GGGGS repeats) exhibited higher in vivo exposure (AUC) but lower in vitro activity than those with longer linkers (2-3 repeats). In vitro activity did not correlate linearly with linker length, as constructs with no linker (n = 0) showed reduced activity, while no consistent trend was observed for n = 1-3. These findings provide valuable insights into the design of hGH-Fc fusion proteins, offering a framework for systematically improving their potency and longevity and supporting the development of long-acting hGH therapies.
Copyright: © 2025 Lee et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Controlled release of human growth hormone fused with a human hybrid Fc fragment through a nanoporous polymer membrane.Nanoscale. 2013 May 21;5(10):4262-9. doi: 10.1039/c3nr00474k. Nanoscale. 2013. PMID: 23546513
-
Insertion of the designed helical linker led to increased expression of tf-based fusion proteins.Pharm Res. 2009 Mar;26(3):523-8. doi: 10.1007/s11095-008-9767-0. Epub 2008 Nov 11. Pharm Res. 2009. PMID: 19002568 Free PMC article.
-
Linker minimization and characterization of Fc-fused interleukin-17A for increased in vivo half-life.Protein Eng Des Sel. 2025 Jan 10;38:gzaf009. doi: 10.1093/protein/gzaf009. Protein Eng Des Sel. 2025. PMID: 40626948
-
[The molecular design and drug development of recombinant long-acting follicle stimulating hormone].Yao Xue Xue Bao. 2012 Apr;47(4):421-6. Yao Xue Xue Bao. 2012. PMID: 22799021 Review. Chinese.
-
Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins.J Pharm Sci. 2015 Jun;104(6):1866-1884. doi: 10.1002/jps.24444. Epub 2015 Apr 14. J Pharm Sci. 2015. PMID: 25872915 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

