Integrating multimodal imaging and peritumoral features for enhanced prostate cancer diagnosis: A machine learning approach
- PMID: 40373044
- PMCID: PMC12080843
- DOI: 10.1371/journal.pone.0323752
Integrating multimodal imaging and peritumoral features for enhanced prostate cancer diagnosis: A machine learning approach
Abstract
Background: Prostate cancer is a common malignancy in men, and accurately distinguishing between benign and malignant nodules at an early stage is crucial for optimizing treatment. Multimodal imaging (such as ADC and T2) plays an important role in the diagnosis of prostate cancer, but effectively combining these imaging features for accurate classification remains a challenge.
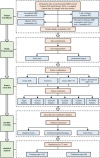
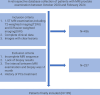
Methods: This retrospective study included MRI data from 199 prostate cancer patients. Radiomic features from both the tumor and peritumoral regions were extracted, and a random forest model was used to select the most contributive features for classification. Three machine learning models-Random Forest, XGBoost, and Extra Trees-were then constructed and trained on four different feature combinations (tumor ADC, tumor T2, tumor ADC+T2, and tumor + peritumoral ADC+T2).
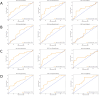
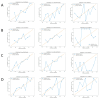
Results: The model incorporating multimodal imaging features and peritumoral characteristics showed superior classification performance. The Extra Trees model outperformed the others across all feature combinations, particularly in the tumor + peritumoral ADC+T2 group, where the AUC reached 0.729. The AUC values for the other combinations also exceeded 0.65. While the Random Forest and XGBoost models performed slightly lower, they still demonstrated strong classification abilities, with AUCs ranging from 0.63 to 0.72. SHAP analysis revealed that key features, such as tumor texture and peritumoral gray-level features, significantly contributed to the model's classification decisions.
Conclusion: The combination of multimodal imaging data with peritumoral features moderately improved the accuracy of prostate cancer classification. This model provides a non-invasive and effective diagnostic tool for clinical use and supports future personalized treatment decisions.
Copyright: © 2025 Zhou et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.The authors have no competing interests to declare that are relevant to the content of this article.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

