Effect of UV-A on endophyte colonisation of Arabidopsis thaliana
- PMID: 40373087
- PMCID: PMC12080771
- DOI: 10.1371/journal.pone.0323576
Effect of UV-A on endophyte colonisation of Arabidopsis thaliana
Abstract
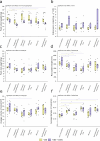
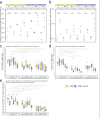
UV-A, an important part of sunlight radiation, is typically absent in experiments on plant-endophyte interactions. We examined the impact of UV-A in the 350-400 nm range (UV-A1 waveband) on the plant interactions with fungal endophytes belonging to different taxonomic groups: Paraphoma chrysanthemicola, Phomopsis columnaris, Diaporthe eres, Mucor sp., and yeast Sporobolomyces ruberrimus. Physiologically relevant levels of UV-A did not substantially affect the colonisation of shoots and roots by endophytes. UV-A upregulated the expression of genes involved in the establishment of symbiosis. Specifically, the expression of PDF1.2 was affected by P. chrysanthemicola and S. ruberrimus only under UV-A conditions. Additionally, UV-A exposure upregulated the mRNA levels of ICS1 and PAL1, genes important for plant responses to stress factors. Inoculation with P. chrysanthemicola and S. ruberrimus led to increased expression of the ICS1 gene. We did not observe significant interactions between the effects of UV-A and the presence of endophytes on other examined plant traits, including plant fresh weight, root system architecture, and expression of plant photoreceptor genes. For these physiological parameters, the effects of the presence of endophytes did not depend on UV-A supplementation. Our findings indicate that while UV-A does not substantially influence plant colonisation by the endophytes, it does trigger the upregulation of plant defence genes and affects the shoot growth of Arabidopsis.
Copyright: © 2025 Giza et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
The effects of fungal root endophytes on plant growth are stable along gradients of abiotic habitat conditions.FEMS Microbiol Ecol. 2018 Feb 1;94(2). doi: 10.1093/femsec/fix162. FEMS Microbiol Ecol. 2018. PMID: 29186430
-
The endophytic fungus Serendipita indica affects auxin distribution in Arabidopsis thaliana roots through alteration of auxin transport and conjugation to promote plant growth.Plant Cell Environ. 2024 Oct;47(10):3899-3919. doi: 10.1111/pce.14989. Epub 2024 Jun 7. Plant Cell Environ. 2024. PMID: 38847336
-
In Vitro Morphogenesis of Arabidopsis to Search for Novel Endophytic Fungi Modulating Plant Growth.PLoS One. 2015 Dec 7;10(12):e0143353. doi: 10.1371/journal.pone.0143353. eCollection 2015. PLoS One. 2015. PMID: 26641657 Free PMC article.
-
Inside the root microbiome: bacterial root endophytes and plant growth promotion.Am J Bot. 2013 Sep;100(9):1738-50. doi: 10.3732/ajb.1200572. Epub 2013 Aug 8. Am J Bot. 2013. PMID: 23935113 Review.
-
Fungal endophytes can modulate plant invasion.Biol Rev Camb Philos Soc. 2024 Oct;99(5):1652-1671. doi: 10.1111/brv.13085. Epub 2024 Apr 17. Biol Rev Camb Philos Soc. 2024. PMID: 38629189 Review.
References
-
- Aphalo P, Albert A, Bjorn L, Mcleod A, Robson T, Rosenqvist E. Beyond the visible: A handbook of best practice in plant UV photobiology. Aphalo P, editor. Helsinki: University of Helsinki. 2012.
-
- Jansen MAK, Biswas DK. Natural variation in UV-B protection amongst Arabidopsis thaliana accessions. EJFA. 2012;24(6). doi: 10.9755/ejfa.v24i6.14681 - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

