Cerebrospinal fluid immune phenotyping reveals distinct immunotypes of myalgic encephalomyelitis/chronic fatigue syndrome
- PMID: 40373264
- PMCID: PMC12311384
- DOI: 10.1093/jimmun/vkaf087
Cerebrospinal fluid immune phenotyping reveals distinct immunotypes of myalgic encephalomyelitis/chronic fatigue syndrome
Abstract
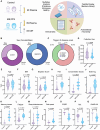
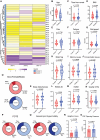
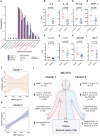
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex heterogeneous multiorgan disease that can have severe impact on individuals' quality of life. Diagnosis of ME/CFS is based on symptom presentation, and a significant goal for the field is to establish meaningful subtypes. The heterogeneity in the literature suggests that individuals living with ME/CFS may suffer from overlapping but different underlying pathophysiological mechanisms. We enrolled 40 participants with ME/CFS and 41 matched healthy control subjects at the Bragée Clinic in Sweden. We assessed plasma samples from both ME/CFS cases and control groups and cerebrospinal fluid (CSF) samples from individuals with ME/CFS. We investigated dysregulated pathways and disease profiles through clinical questionnaires; multiplex analyses of cytokines, hormones, and matrix metalloproteinases; pathogen seroreactivity through peptide display bacteria libraries; and high-throughput microarray for autoantibodies. All samples used were from humans. We show altered interaction patterns between circulating biological factors in plasma of ME/CFS participants. Our analysis of CSF from individuals with ME/CFS revealed different immunotypes of disease. We found 2 patient clusters based on matrix metalloproteinases profiles. The subgroups had similar clinical presentation but distinct pathogen exposure and CSF inflammatory profiles. Our findings shed light on ME/CFS immune phenotypes and generate hypotheses for future research in disease pathogenesis and treatment development by exploring disease subgroups.
Keywords: ME/CFS; cerebrospinal fluid; immune phenotypes; matrix metalloproteinases; neuroimmunology.
© The Author(s) 2025. Published by Oxford University Press on behalf of The American Association of Immunologists.
Conflict of interest statement
A.I. co-founded RIGImmune, Xanadu Bio, and PanV and is a member of the Board of Directors of Roche Holding Ltd and Genentech. All other authors declare no competing interests.
Figures

References
-
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. National Academies Press; 2015. - PubMed
-
- Bateman L et al. Chronic fatigue syndrome and co-morbid and consequent conditions: evidence from a multi-site clinical epidemiology study. Fatigue Biomed Health Behav. 2015;3:1–15.
-
- Ortega-Hernandez O, Shoenfeld Y. Infection, vaccination, and autoantibodies in chronic fatigue syndrome, cause or coincidence? Ann N Y Acad Sci. 2009;1173:600–609. - PubMed
-
- Chia J, Chia A, Voeller M, Lee T, Chang R. Acute enterovirus infection followed by myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and viral persistence: figure 1. J Clin Pathol. 2010;63:165–168. - PubMed
MeSH terms
Substances
Grants and funding
- Howard Hughes Medical Institute Collaborative COVID-19 Initiative
- 88887.695252/2022-00/Brazilian Federal Agency for Support and Evaluation of Graduate Education
- Poly-Bio Research Foundation
- R01AI157488/National Institute of Allergy and Infectious Diseases
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Medical

