Coronary Plaque, Inflammation, Subclinical Myocardial Injury, and Major Adverse Cardiovascular Events in the REPRIEVE Substudy
- PMID: 40373520
- PMCID: PMC12138200
- DOI: 10.1016/j.jacadv.2025.101781
Coronary Plaque, Inflammation, Subclinical Myocardial Injury, and Major Adverse Cardiovascular Events in the REPRIEVE Substudy
Abstract
Background: In REPRIEVE (Randomized Trial to Prevent Vascular Events in HIV), pitavastatin prevented major adverse cardiovascular events (MACE) and reduced noncalcified coronary plaque (NCP) among people with HIV and low-to-moderate traditional cardiovascular disease (CVD) risk.
Objectives: The purpose of this study was to assess the relationship of coronary plaque, inflammation, and subclinical myocardial injury with MACE.
Methods: A total of 804 REPRIEVE Mechanistic Substudy participants enrolled from April 2015 to February 2018 at 31 U.S. sites, randomized to pitavastatin 4 mg/day or placebo, and followed for incident MACE (median 6.2 years [Q1-Q3 5.4-7.1]), were assessed for relationships of baseline NCP, markers of inflammation (high-sensitivity C-reactive protein [hs-CRP], interleukin (IL)-6, oxidized low-density lipoprotein, and lipoproprotein-associated phospholipase A2), and subclinical myocardial injury (high-sensitivity cardiac troponin T [hs-cTnT]) with MACE.
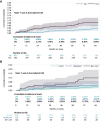
Results: Among enrolled participants (17% female [139/804], 47% non-White [379/804], median age 51 years, median low-density lipoprotein 105 mg/dL, 10-year atherosclerotic CVD [ASCVD] risk 4.6%, 40% [299/755] with noncalcified plaque), MACE incidence was 7.26/1,000 (95% CI: 4.51-11.7) person-years (17 events) for pitavastatin and 9.15/1,000 person-years (95% CI: 5.97-14.0) (21 events) for placebo. The hazard of MACE was greater in those with (vs without) noncalcified plaque (HR: 2.5; [95% CI: 1.3-4.8]; P = 0.008), with higher levels of hs-CRP (P = 0.049), IL-6 (P = 0.033), and hs-cTnT (P = 0.003) at study entry, persisting after ASCVD risk adjustment. In exploratory prediction modeling, adding hs-CRP, IL-6, and hs-cTnT to ASCVD risk increased the integrated area under the curve to 0.72 and C-statistic to 0.73 (0.62-0.84) vs 0.58 and 0.56 (0.45-0.67) compared to ASCVD risk alone.
Conclusions: NCP and higher hs-CRP, IL-6, and hs-cTnT were associated with MACE and improved risk prediction over traditional risk factors in people with HIV without cardiac symptoms and low-to-moderate ASCVD risk. (Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults [REPRIEVE]; NCT02344290).
Keywords: HIV; biomarkers; coronary plaque; inflammation; major adverse cardiovascular events; myocardial injury.
Copyright © 2025 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding support and author disclosures This study is supported through NIH grants U01HL123336 and 1UG3HL164285, to the Clinical Coordinating Center, and U01HL123339 and 1U24HL164284, to the Data Coordinating Center, as well as funding from Kowa Pharmaceuticals America, Inc, Gilead Sciences, and ViiV Healthcare. The NIAID supported this study through grants UM1 AI068636, which supports the ACTG Leadership and Operations Center; and UM1 AI106701, which supports the ACTG Laboratory Center. This work was also supported by the Nutrition Obesity Research Center at Harvard (P30DK040561 to Dr Grinspoon). The views expressed in this paper are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute or the National Institute of Allergy and Infectious Diseases; the National Institutes of Health; or the U.S. Department of Health and Human Services. Dr Lu has received grant support through his institution from the NIH/NHLBI and Kowa Pharmaceuticals America for the conduct of the study; and also has received research support to his institution from the American Heart Association, AstraZeneca, Ionis, Johnson & Johnson Innovation, MedImmune, the National Academy of Medicine, the NIH/NHLBI, and the Risk Management Foundation of the Harvard Medical Institutions Incorporated outside of the submitted work. Dr Ribaudo has received grants from Kowa Pharmaceuticals during the conduct of the study, as well as grants from NIH/NIAID, NIH/NHLBI, NIH/NIDDK, and NIH/NIA, outside of the submitted work. Dr Zanni has received grant support through her institution from NIH/NIAID and Gilead Sciences, Inc, relevant to the conduct of the study, as well as grants from NIH/NIAID and NIH/NHLBI; support for attending CROI and International Workshop for HIV and Women from conference organizing committee when abstract reviewer and/or speaker; and participation in DSMB for NIH funded studies, outside the submitted work. Dr deFilippi has received grants from Massachusetts General Hospital outside the submitted work; has received grants/contracts from Abbott Diagnostics, FujiRebio, Quidel:Ortho, Randox, Roche Diagnostics, and Siemens Healthineers outside this submitted work; and also has received consulting income from Abbott Diagnostics, Polymedco, Quidel:Ortho, Roche Diagnostics, Siemens Healthineers, and Tosoh. Dr Taron has received grants from the Deutsche Forschungsgesellschaft (German Research Foundation, TA 1438/1-2) during the conduct of the study as well as personal fees from Universimed Cross Media Content, coreLab Black Forest, and Onc AI outside the submitted work. Dr Foldyna has received grants from the National Heart, Lung, and Blood Institute, AstraZeneca, MedImmune, MedTrace, and Cleerly Health outside the submitted work. Dr Burdo has received grants from Massachusetts General Hospital during the conduct of the study and is a member of the scientific advisory board and has equity in Excision BioTherpeutics, outside the submitted work. Dr Currier has received consulting fees from Merck and Company, outside the submitted work. Dr Fichtenbaum has received research grant support through his institution from Gilead Sciences, ViiV Healthcare, GSK, and Merck, all outside the submitted work. Dr Malvestutto has received institutional research support by Lilly and honoraria from ViiV Healthcare, Gilead Sciences, and Pfizer for advisory board membership, all outside the submitted work. Dr Aberg has received grants from Massachusetts General Hospital during the conduct of the study; has received institutional research support for clinical trials from Emergent BioSolutions, Gilead Sciences, GlaxoSmithKline, Janssen, Merck, Pfizer, Regeneron, and ViiV Healthcare, and has received personal fees for advisory boards from GlaxoSmithKline/ViiV, Invivyd, Merck and Regeneron; and participation on DSMB for Kintor Pharmaceuticals, all outside the submitted work. Dr Grinspoon has received grant support through his institution from NIH, Kowa Pharmaceuticals America, Inc, Gilead Sciences, Inc, and ViiV Healthcare for the conduct of the study; has received personal fees from Theratechnologies and ViiV; and has served on the scientific advisory board of Marathon Asset Management and Exavir Therapeutics, all outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




References
Associated data
Grants and funding
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- K24 AI157882/AI/NIAID NIH HHS/United States
- U01 HL123339/HL/NHLBI NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- UG3 HL164285/HL/NHLBI NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 HL123336/HL/NHLBI NIH HHS/United States
- U24 HL164284/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

