What is the current evidence base for measles vaccination earlier than 9 months of age?: Report from an informal technical consultation of the World Health Organization
- PMID: 40373694
- PMCID: PMC12176663
- DOI: 10.1016/j.vaccine.2025.127187
What is the current evidence base for measles vaccination earlier than 9 months of age?: Report from an informal technical consultation of the World Health Organization
Abstract
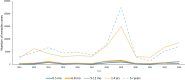
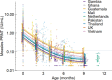
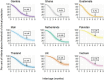
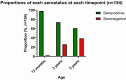
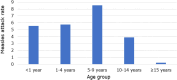
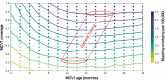
Measles is one of the most contagious vaccine preventable diseases, causing severe complications and deaths globally. While vaccination with a measles-containing vaccine (MCV) has prevented millions of measles deaths, recent trends, especially from low- and middle-income countries, are discouraging. Measles cases have increased since 2021 as MCV coverage has decreased; and an estimated 107,500 measles deaths, mostly in children under-five years, occurred in 2023. Thus, a renewed focus on proven and innovative strategies to control measles is needed. The World Health Organization (WHO) recommends a first MCV dose administered at 9-15 months of age (routine MCV1), however MCV1 below 9 months of age (early MCV1) may increase vaccination coverage because uptake of all vaccines tends to be higher the younger the child, and this might protect vulnerable infants earlier in life. However, due to concerns about possible reduced vaccine performance, early MCV1 is not routinely recommended by WHO. WHO hosted an informal technical consultation on December 6-7, 2023, in Geneva, Switzerland to evaluate recent evidence on early MCV1 and identify evidence gaps for policy making. The recent evidence suggests a robust humoral immune response shortly after early MCV1 at 5-8 months of age. Immune blunting of a routine second MCV dose (e.g., MCV2) after early MCV1 was not demonstrated in the presented data. However, 3-7 years after MCV1, children receiving early MCV1 had lower measles antibodies than children receiving routine MCV1, suggesting faster waning of immunity. The totality of evidence on immune blunting remains inconsistent. Meeting participants thought more data are needed before revisiting WHO's current recommendation for a potential revision. Evidence gaps include: understanding measles disease burden and severity in infants; early MCV1 effectiveness and duration; vaccine-induced cellular immunogenicity; whether measles in infants is acquired from other infants or older children or adults; and blunting of routine MCV2. Addressing evidence gaps through targeted studies and measles outbreak investigations, as well as evaluations of country-level introductions of early MCV1 are warranted. Ensuring high MCV1 and MCV2 coverage remains the priority in measles control.
Keywords: Early measles vaccination; Immune blunting; Immunogenicity; Vaccine effectiveness.
Copyright © 2025. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Authors and observers who attended the WHO meeting were subject to declaring potential competing interests. The following authors declared potential competing interests: Arnaud M. Didierlaurent is a previous employee of GlaxoSmithKline, data safety and monitoring member for AMC Biologicals, consultant for Sanofi, and speaker for Sanofi and Merck. He is a member of the Emergency Use Listing WHO Technical Advisory Group. His current research unit has research collaboration agreements with Moderna, Sanofi, and GSK; Dorthe Maria Vittrup receives financial compensation for academic teaching and public speaking on measles; L. Kendall Krause, Kevin McCarthy, Kristen Earle, and Kurt Frey are employed by the Bill & Melinda Gates Foundation which funded the meeting; Shelly Bolotin is employed by public sector entities with an interest in vaccine research and surveillance. She serves as the Director of the Centre for Vaccine Preventable Diseases at the University of Toronto. The center has received donations from Merck, Pfizer and Sanofi and adheres to governance processes at the University of Toronto to ensure it operates independently. As the Director, she has advocated for increasing immunization coverage and has publicly made statements about the safety and effectiveness of specific vaccines, including MCV. She has been funded by various Canadian Federal and other initiatives; Walter A. Orenstein is a consultant for Sanofi. The following authors declared no potential competing interests: Daniel Kapelus; Gaston De Serres; Merryn Voysey; Rob van Binnendijk; Susan Hahné; Tom Woudenberg; William J. Moss. The following observers declared potential competing interests: Ana Leticia Nery is employed by the Bill & Melinda Gates Foundation which funded this meeting; Anthony Scott receives research support for measles-related work from the Medical Research Council of the United Kingdom and Bill & Melinda Gates Foundation; Gerald Bright Businge receives research support for measles-related work from the Bill & Melinda Gates Foundation. The following observers declared no potential competing interests: Lien Anh Ha Do; Joy Lee; Katrina Kretsinger.
Figures


References
-
- World Health Organization. Measles [Internet] 2024. https://www.who.int/news-room/fact-sheets/detail/measles [cited 2024 Jul 5]. Available from:
-
- WHO . 2023. Internal analysis of progress towards measles and / or rubella elimination based on the most recent report submitted by the National Verification Committee (NVC) and evaluated by the Regional Verification Commission (RVC)
-
- WHO . 2023. Internal analysis of large or disruptive measles outbreaks in the 12 months to June 2023 based on country-specific data received in August 2023 covering the period between 2022–07 and 2023–06 and data from the World Population prospects 2019 revision.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

