A comprehensive engineering strategy improves potency and manufacturability of a near pan-neutralizing antibody against HIV
- PMID: 40373766
- PMCID: PMC12227296
- DOI: 10.1016/j.str.2025.04.016
A comprehensive engineering strategy improves potency and manufacturability of a near pan-neutralizing antibody against HIV
Abstract
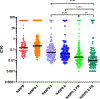
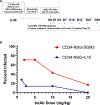
Anti-HIV envelope broadly neutralizing antibodies (bnAbs) are alternatives to conventional antiretrovirals with the potential to prevent and treat infection, reduce latent reservoirs, and/or mediate a functional cure. Clinical trials with "first-generation" bnAbs used alone or in combination show promising antiviral effects but also highlight that additional engineering of "enhanced" antibodies will be required for optimal clinical utility, while preserving or enhancing Current Good Manufacturing Practices (cGMP) manufacturing capability. Here, we report the engineering of an anti-CD4-binding site (CD4bs) bnAb, N49P9.3. Through a series of rational modifications, we produced a variant, N49P9.6-FR-LS, that demonstrates enhanced potency, superior antiviral activity in combination with other bnAbs, low polyreactivity, and longer circulating half-life. Additional engineering for manufacturing produced a final variant, eN49P9, with properties conducive to cGMP production. Overall, these efforts demonstrate the feasibility of developing enhanced anti-CD4bs bnAbs with greatly improved antiviral properties as well as potential translational value.
Keywords: ADCC; CD4 binding site; HIV; HIV cure; bnAb; combination therapies; elite neutralizer; engineering; monoclonal antibody; neutralization.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Update of
-
A comprehensive engineering strategy improves potency and manufacturability of a near pan-neutralizing antibody against HIV.bioRxiv [Preprint]. 2024 Oct 17:2024.10.14.618178. doi: 10.1101/2024.10.14.618178. bioRxiv. 2024. Update in: Structure. 2025 Jul 3;33(7):1150-1164.e8. doi: 10.1016/j.str.2025.04.016. PMID: 39464103 Free PMC article. Updated. Preprint.
References
-
- Bigoloni A, Gianotti N, Spagnuolo V, Galli L, Nozza S, Cossarini F, Salpietro S, Carini E, Piatti P, Vinci C, et al. (2012). Long-term glucose tolerance in highly experienced HIV-infected patients receiving nucleoside analogue-sparing regimens. AIDS 26, 1837–1840. 10.1097/QAD.0b013e32835705dd. - DOI - PubMed
-
- Dirajlal-Fargo S, Moser C, Brown TT, Kelesidis T, Dube MP, Stein JH, Currier J, and McComsey GA (2016). Changes in Insulin Resistance After Initiation of Raltegravir or Protease Inhibitors With Tenofovir-Emtricitabine: AIDS Clinical Trials Group A5260s. Open Forum Infect. Dis 3, ofw174. 10.1093/ofid/ofw174. - DOI - PMC - PubMed
-
- Katlama C, Assoumou L, Valantin MA, Soulié C, Martinez E, Béniguel L, Bouchaud O, Raffi F, Molina JM, Fellahi S, et al. (2019). Dual therapy combining raltegravir with etravirine maintains a high level of viral suppression over 96 weeks in long-term experienced HIV-infected individuals over 45 years on a PI-based regimen: results from the Phase II ANRS 163 ETRAL study. J. Antimicrob. Chemother 74, 2742–2751. 10.1093/jac/dkz224. - DOI - PubMed
-
- Spieler G, Westfall AO, Long DM, Cherrington A, Burkholder GA, Funderburg N, Raper JL, Overton ET, and Willig AL (2022). Trends in diabetes incidence and associated risk factors among people living with HIV in the current treatment era. AIDS 36, 1811–1818. 10.1097/QAD.0000000000003348. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

