NSP6 regulates calcium overload-induced autophagic cell death and is regulated by KLHL22-mediated ubiquitination
- PMID: 40373961
- PMCID: PMC12302427
- DOI: 10.1016/j.jare.2025.05.031
NSP6 regulates calcium overload-induced autophagic cell death and is regulated by KLHL22-mediated ubiquitination
Abstract
Introduction: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) poses a substantial global threat. SARS-CoV-2 nonstructural proteins (NSPs) are essential for impeding the host replication mechanism while also assisting in the production and organization of new viral components. However, NSPs are not incorporated into viral particles, and their subsequent fate within host cells remains poorly understood. Additionally, their role in viral pathogenesis requires further investigation.
Objectives: This study aimed to discover the ultimate fate of NSP6 in host cells and to elucidate its role in viral pathogenesis.
Methods: We investigated the effects of NSP6 on cell death and explored the underlying mechanism; moreover, we examined the degradation mechanism of NSP6 in human cells, along with analysing its correlation with coronavirus disease 2019 (COVID-19) severity in patient peripheral blood mononuclear cells (PBMCs).
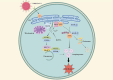
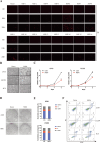
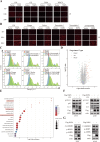
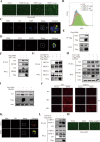
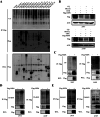
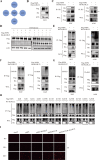
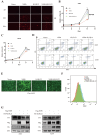
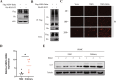
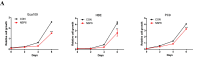
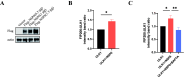
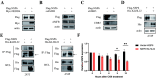
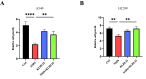
Results: NSP6 was demonstrated to induce cell death. Specifically, NSP6 interacted with EI24 autophagy-associated transmembrane protein (EI24) to increase intracellular Ca2+ levels, thereby enhancing the interactions between unc-51-like autophagy activating kinase 1 (ULK1) and RB1 inducible coiled-coil 1 (RB1CC1/FIP200), as well as beclin 1 (BECN1) and phosphatidylinositol 3-kinase catalytic subunit type 3 (PIK3C3). This cascade ultimately triggers autophagy, thus resulting in cell death. Additionally, we discovered that the homeostasis of the NSP6 protein was regulated by K48-linked ubiquitination. We identified kelch-like protein 22 (KLHL22) as the E3 ligase that was responsible for ubiquitinating and degrading NSP6, restoring intracellular calcium homeostasis and reversing NSP6-induced autophagic cell death. Moreover, NSP6 expression levels were observed to be positively associated with the severity of SARS-CoV-2-induced disease.
Conclusion: This study reveals that KLHL22-mediated ubiquitination controls NSP6 stability and that NSP6 induces autophagic cell death via calcium overload, highlighting its cytotoxic role and suggesting therapeutic strategies that target calcium signaling or promote NSP6 degradation as potential interventions against COVID-19.
Keywords: Autophagy; Calcium overload; KLHL22; NSP6; SARS-CoV-2; Ubiquitination.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
The NSP6-L260F substitution in SARS-CoV-2 BQ.1.1 and XBB.1.16 lineages compensates for the reduced viral polymerase activity caused by mutations in NSP13 and NSP14.J Virol. 2025 Jun 17;99(6):e0065625. doi: 10.1128/jvi.00656-25. Epub 2025 May 13. J Virol. 2025. PMID: 40358207 Free PMC article.
-
Potassium molybdate blocks APN-dependent coronavirus entry by degrading receptor via PIK3C3-mediated autophagy.J Virol. 2025 Jan 31;99(1):e0144924. doi: 10.1128/jvi.01449-24. Epub 2024 Dec 6. J Virol. 2025. PMID: 39641621 Free PMC article.
-
Artificial targeting of autophagy components to mitochondria reveals both conventional and unconventional mitophagy pathways.Autophagy. 2025 Feb;21(2):315-337. doi: 10.1080/15548627.2024.2395149. Epub 2024 Sep 8. Autophagy. 2025. PMID: 39177530 Free PMC article.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

