Effect of N-Glycan Profiles on Binding Affinity of Diagnostic Antibody Produced by Hybridomas in Serum-Free Suspension
- PMID: 40374547
- PMCID: PMC12099623
- DOI: 10.4014/jmb.2501.01036
Effect of N-Glycan Profiles on Binding Affinity of Diagnostic Antibody Produced by Hybridomas in Serum-Free Suspension
Abstract
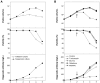
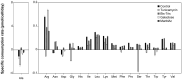
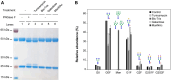
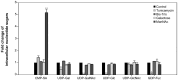
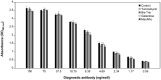
Serum-free suspension culture for hybridomas is one of the important key steps for efficient diagnostic antibody production while maintaining protein quality and function. Based on the importance of N-glycan profiles in therapeutic antibody production in mammalian cells, the effect of changes in the N-glycan profiles on the function of diagnostic antibody must also be validated. To investigate the influence of diagnostic antibodies with different N-glycan profiles on the binding affinity with target antigens, four glycosylation regulators, tunicamycin, Bis-Tris, galactose, and N-acetylmannosamine, were administered separately to diagnostic antibody-producing hybridomas cultures. Supplementation with these four glycosylation modulators inhibited glycosylation and increased mannosylation, galactosylation, and sialylation in serum-free suspended hybridomas. In particular, the diagnostic antibody produced from a culture with tunicamycin exhibited a significant increase in the aglycosylated form compared with those without tunicamycin or with other glycosylation modulators. Surprisingly, diagnostic antibody with different N-glycan compositions did not significantly affect binding affinity with the target antigen and even aglycosylated antibodies did not affect binding affinity. Taken together, the results indicate that the change in the N-glycan profile of the diagnostic antibody produced in serum-free suspension hybridomas in an altered culture environment did not significantly affect their biological function, which provides valuable insight for the production and quality control of diagnostic antibody.
Keywords: Hybridomas; binding affinity; diagnostic antibody; glycosylation; suspension culture.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures
References
-
- Sait A, Korkmaz S, Parmaksız A, Erbaş O. Hybridoma technology for the production of monoclonal antibodies. D J. Med. Sci. 2022;8:141–149.
-
- Bilodeau A. The pros and cons of adherent versus suspension cell culture. Biopharm. Int. 2024;37:18–22.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

