Autophagy- and oxidative stress-related protein deregulation mediated by extracellular vesicles of human MJD/SCA3 iPSC-derived neuroepithelial stem cells and differentiated neural cultures
- PMID: 40374597
- PMCID: PMC12081669
- DOI: 10.1038/s41419-025-07659-0
Autophagy- and oxidative stress-related protein deregulation mediated by extracellular vesicles of human MJD/SCA3 iPSC-derived neuroepithelial stem cells and differentiated neural cultures
Abstract
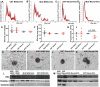
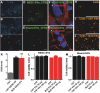
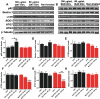
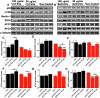
Extracellular vesicles (EVs) have been associated with the transport of molecules related to the pathological processes in neurodegenerative diseases. Machado-Joseph disease (MJD) is a neurodegenerative disorder triggered by mutant ataxin-3 protein that causes protein misfolding and aggregation resulting in neuronal death. To evaluate EVs' role in the potential spread of disease-associated factors in MJD, in this study, EVs were isolated from human Control (CNT) and MJD induced-pluripotent stem cell-derived neuroepithelial stem cells (iPSC-derived NESC) and their differentiated neural cultures (cell cultures composed of neurons and glia). EVs were characterized and investigated for their ability to interfere with cell mechanisms known to be impaired in MJD. The presence of mRNA and proteins related to autophagy, cell survival, and oxidative stress pathways, and the mutant ataxin-3, was evaluated in the EVs. SOD1, p62, and Beclin-1 were found present both in CNT and MJD EVs. Lower levels of the p62 autophagy-related protein and higher levels of the oxidative stress-related SOD1 protein were found in MJD EVs. The oxidative stress-related CYCS mRNA and autophagy-related SQSTM1, BECN1, UBC, ATG12, and LC3B mRNAs were detected in EVs and no significant differences in their levels were observed between CNT and MJD EVs. The internalization of EVs by human CNT neurons was demonstrated, and no effect of the EVs administration was observed on cell viability. Moreover, the incubation of MJD EVs (isolated from NESC or differentiated neural cultures) with human CNT differentiated neural cells resulted in the reduction of SOD1 and autophagy-related proteins ATG3, ATG7, Beclin-1, LC3B, and p62 levels. Finally, a tendency for accumulation of ataxin-3-positive aggregates in CNT differentiated neural cells co-cultured with MJD differentiated neural cells was observed. Overall, our data indicate that EVs carry autophagy- and oxidative stress-related proteins and mRNAs and provide evidence of MJD EVs-mediated interference with autophagy and oxidative stress pathways.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: Human fibroblast isolation, for iPSC-derived NESC generation, was performed with informed consent obtained from all participants and was approved by the Ethics Committee of the Medical Faculty of the University of Coimbra, Portugal (Ref. CE-152/2011), and all methods were performed in accordance with the relevant guidelines and regulations, as previously described [57].
Figures


Similar articles
-
Fibroblasts of Machado Joseph Disease patients reveal autophagy impairment.Sci Rep. 2016 Jun 22;6:28220. doi: 10.1038/srep28220. Sci Rep. 2016. PMID: 27328712 Free PMC article.
-
Calpain Inhibition Is Protective in Machado-Joseph Disease Zebrafish Due to Induction of Autophagy.J Neurosci. 2017 Aug 9;37(32):7782-7794. doi: 10.1523/JNEUROSCI.1142-17.2017. Epub 2017 Jul 7. J Neurosci. 2017. PMID: 28687604 Free PMC article.
-
Autophagy Promoted the Degradation of Mutant ATXN3 in Neurally Differentiated Spinocerebellar Ataxia-3 Human Induced Pluripotent Stem Cells.Biomed Res Int. 2016;2016:6701793. doi: 10.1155/2016/6701793. Epub 2016 Oct 25. Biomed Res Int. 2016. PMID: 27847820 Free PMC article.
-
Autophagy in Spinocerebellar Ataxia Type 3: From Pathogenesis to Therapeutics.Int J Mol Sci. 2023 Apr 17;24(8):7405. doi: 10.3390/ijms24087405. Int J Mol Sci. 2023. PMID: 37108570 Free PMC article. Review.
-
Molecular Mechanisms and Cellular Pathways Implicated in Machado-Joseph Disease Pathogenesis.Adv Exp Med Biol. 2018;1049:349-367. doi: 10.1007/978-3-319-71779-1_18. Adv Exp Med Biol. 2018. PMID: 29427113 Review.
References
-
- Paulson HL, Perez MK, Trottier Y, Trojanowski JQ, Subramony SH, Das SS, et al. Intranuclear inclusions of expanded polyglutamine protein in spinocerebellar ataxia type 3. Neuron. 1997;19:333–44. - PubMed
-
- Scherzed, Brunt W, Heinsen ER, de Vos RA H, Seidel K, Bürk K, et al. Pathoanatomy of cerebellar degeneration in spinocerebellar ataxia type 2 (SCA2) and type 3 (SCA3). Cerebellum. 2012;11:749–60. - PubMed
-
- Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rüb U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012;124:1–21. - PubMed
-
- Kawaguchi Y, Okamoto T, Taniwaki M, Aizawa M, Inoue M, Katayama S, et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994;8:221–8. - PubMed
-
- Matos CA, de Macedo-Ribeiro S, Carvalho AL. Polyglutamine diseases: the special case of ataxin-3 and Machado-Joseph disease. Prog Neurobiol. 2011;95:26–48. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

