Identification of tRF-29-79MP9P9NH525 as a biomarker and tumor suppressor of gastric cancer via regulating KIF14/AKT pathway
- PMID: 40374610
- PMCID: PMC12081660
- DOI: 10.1038/s41420-025-02514-9
Identification of tRF-29-79MP9P9NH525 as a biomarker and tumor suppressor of gastric cancer via regulating KIF14/AKT pathway
Erratum in
-
Correction to: Identification of tRF-29-79MP9P9NH525 as a biomarker and tumor suppressor of gastric cancer via regulating KIF14/AKT pathway.Cell Death Discov. 2025 Jul 30;11(1):353. doi: 10.1038/s41420-025-02614-6. Cell Death Discov. 2025. PMID: 40739085 Free PMC article. No abstract available.
Abstract
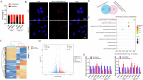
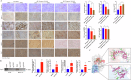
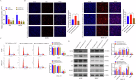
Gastric cancer (GC) is one of the most common malignancies with a poor prognosis. The development of novel biomarkers is of utmost importance to screen patients with GC. Molecular mechanism study of GC may provide a research basis for the development of targeted drugs. We identified tRF-29-79MP9P9NH525 (tRF-29) as a GC-associated tRNA-derived fragment (tRF). The specific hair-pin structure reverse primer and amplification primers were first designed and then applied for tRF-29 quantification. Receiver operator characteristic curve, Kaplan-Meier survival curve, and multivariate Cox analysis were applied to analyze the diagnostic and prognostic values of tRF-29 in GC. Ethynyl-2'-deoxyuridine, cell cloning, Transwell assay, and flow cytometry were used to detect the effects of tRF-29 on proliferation, migration, and cell cycle distribution of GC cells. Xenograft tumor formation in NOD-SCID mice was applied in determining tRF-29's effects on tumor growth. Fluorescence in situ hybridization, dual luciferase reporter assay, Western blot, immunohistochemistry, and RNA-binding protein immunoprecipitation were conducted to explore the molecular mechanism underlying tRF-29 regulating GC development. It was found that tissue tRF-29 showed effective diagnostic efficiency in GC and could discriminate different gastric mucosa. Besides, plasma tRF-29 improved GC diagnostic values of common tumor markers and had prognostic values in GC. tRF-29 was found to suppress proliferation and cell cycle progression. tRF-29 inhibited the growth of xenograft tumors. Mechanically, tRF-29 exerted Kinesin family member 14 (KIF14) mRNA destabilization by combining with argonaute 2 (Ago2) and regulated AKT/P27 pathway. In conclusion, tRF-29 inhibited GC progression by combining with Ago2 and regulated AKT/P27 pathway by silencing KIF14 expression. In normal cells, tRF-29, derived from tRNA-ValACC, targets the 3'UTR region of KIF14 mRNA by forming RNA silencing complex with Ago2. Reduced KIF14 results in less phospholation of AKT. Subsequently, the expression of P27 is increased, while the expression of MMP-2 is decreased. Finally, the cell cycle is arrested, and the cell proliferation is suppressed, as well as the metastasis is inhibited. In gastric cancer cells, due to the downregulated of tRF-29, the expression of KIF14 is increased, thus the cell proliferation and metastasis are promoted via AKT pathway.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The investigation protocol granted approval from the Ethics Committee of Ningbo University (approval no. 2019022501) and was performed in adherence to the ethical principles outlined in the Declaration of Helsinki for medical research including human beings. Before being included in this study, all individuals were required to provide written informed consent. The protocol for the treatment of animals was approved by the Ningbo University Animal Ethics Committee (approval no. 2019-57) in accordance with the Declaration of Basel. Consent for publication: All authors are in agreement with the publication of the manuscript.
Figures





Similar articles
-
A novel tsRNA, tRF-33-6978WPRLXN4V0O inhibits breast cancer development via regulating PTEN/AKT pathway in BHLHA15-mediated manner.J Mol Med (Berl). 2025 Sep;103(9):1135-1148. doi: 10.1007/s00109-025-02566-5. Epub 2025 Jul 28. J Mol Med (Berl). 2025. PMID: 40719768
-
Clinical Diagnostic Values of Transfer RNA-Derived Fragment tRF-41-YDLBRY73W0K5KKOVD and its Effects on the Growth of Gastric Cancer Cells.DNA Cell Biol. 2023 Mar;42(3):176-187. doi: 10.1089/dna.2022.0495. Epub 2023 Mar 3. DNA Cell Biol. 2023. PMID: 36867154
-
tRF-Pro-CGG Suppresses Cell Proliferation and Promotes Apoptosis in Pancreatic Cancer.Dig Dis Sci. 2025 Jun;70(6):2043-2053. doi: 10.1007/s10620-025-08943-x. Epub 2025 Mar 8. Dig Dis Sci. 2025. PMID: 40056302
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
-
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2024;74:229–63. - PubMed
-
- Patel TH, Cecchini M. Targeted therapies in advanced gastric cancer. Curr Treat Options Oncol. 2020;21:70. - PubMed
-
- Doi T, Shitara K, Naito Y, Shimomura A, Fujiwara Y, Yonemori K, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18:1512–22. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

