Photoexcited nitroarene-enabled carbon chain-elongated oxidation of alkenes via tandem oxidative cleavage and dipolar cycloaddition
- PMID: 40374615
- PMCID: PMC12081768
- DOI: 10.1038/s41467-025-59274-4
Photoexcited nitroarene-enabled carbon chain-elongated oxidation of alkenes via tandem oxidative cleavage and dipolar cycloaddition
Abstract
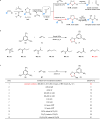
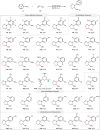
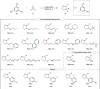
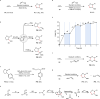
Oxidation of alkenes with O3 and photoexcited nitroarenes represents one of the most attractive organic chemical transformations for the synthesis of oxygen-enriched molecules. However, known achievements are mainly limited to carbon chain-shortened oxidation and carbon chain-retained oxidation of alkenes. Given that constructing higher molecular complexity is the core goal of modern synthesis, the development of chain-elongated oxidation of alkenes would be in high demand but still remains an elusive challenge so far. Herein, we report a photoexcited nitroarene-enabled highly regioselective chain-elongated oxidation of alkenes via tandem oxidative cleavage and dipolar cycloaddition, providing a broad range of synthetically-useful isoxazolidines in up to 92% yield from readily available enol ethers or styrene and derivatives under simple and mild conditions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Urgoitia, G., SanMartin, R., Herrero, M. T. & Domínguez, E. Aerobic cleavage of alkenes and alkynes into carbonyl and carboxyl compounds. ACS Catal.7, 3050–3060 (2017).
-
- Kerenkan, A. E., Bélanda, F. & Do, T.-O. Chemically catalyzed oxidative cleavage of unsaturated fatty acids and their derivatives into valuable products for industrial applications: a review and perspective. Catal. Sci. Technol.6, 971–987 (2016).
-
- Rajagopalan, A., Lara, M. & Kroutil, W. Oxidative alkene cleavage by chemical and enzymatic methods. Adv. Synth. Catal.355, 3321–3335 (2013).
-
- Biermann, U., Bornscheuer, U., Meier, M. A. R., Metzger, J. O. & Schäfer, H. J. Oils and fats as renewable raw materials in chemistry. Angew. Chem. Int. Ed.50, 3854–3871 (2011). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

