TIGIT deficiency promotes autoreactive CD4+ T-cell responses through a metabolic‒epigenetic mechanism in autoimmune myositis
- PMID: 40374622
- PMCID: PMC12081758
- DOI: 10.1038/s41467-025-59786-z
TIGIT deficiency promotes autoreactive CD4+ T-cell responses through a metabolic‒epigenetic mechanism in autoimmune myositis
Abstract
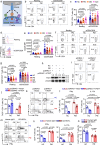
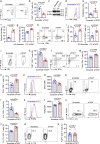
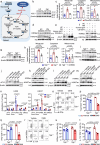
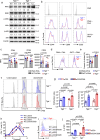
Polymyositis (PM) is a systemic autoimmune disease characterized by muscular inflammatory infiltrates and degeneration. T-cell immunoreceptor with Ig and ITIM domains (TIGIT) contributes to immune tolerance by inhibiting T cell-mediated autoimmunity. Here, we show that a reduced expression of TIGIT in CD4+ T cells from patients with PM promotes these cells' differentiation into Th1 and Th17 cells, which could be rescued by TIGIT overexpression. Knockout of TIGIT enhances muscle inflammation in a mouse model of experimental autoimmune myositis. Mechanistically, we find that TIGIT deficiency enhances CD28-mediated PI3K/AKT/mTOR co-stimulatory pathway, which promotes glucose oxidation, citrate production, and increased cytosolic acetyl-CoA levels, ultimately inducing epigenetic reprogramming via histone acetylation. Importantly, pharmacological inhibition of histone acetylation suppresses the differentiation of Th1 and Th17 cells, alleviating muscle inflammation. Thus, our findings reveal a mechanism by which TIGIT directly affects the differentiation of Th1 and Th17 T cells through metabolic‒epigenetic reprogramming, with important implications for treating systemic autoimmune diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
- 82402113/National Natural Science Foundation of China (National Science Foundation of China)
- 82471764/National Natural Science Foundation of China (National Science Foundation of China)
- 92474103/National Natural Science Foundation of China (National Science Foundation of China)
- 82071819/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

