Chemotactic Zn micromotor for treatment of high blood ammonia-associated hepatic encephalopathy
- PMID: 40374637
- PMCID: PMC12081644
- DOI: 10.1038/s41467-025-59650-0
Chemotactic Zn micromotor for treatment of high blood ammonia-associated hepatic encephalopathy
Abstract
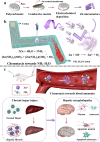
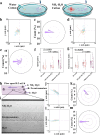
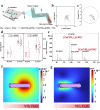
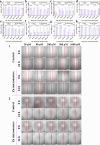
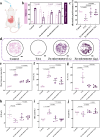
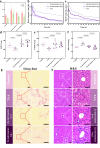
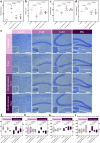
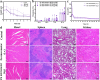
Hepatic fibrosis involves hepatocyte damage, causing blood ammonia accumulation, which exacerbates liver pathology and crosses the blood-brain barrier, inducing hepatic encephalopathy. It is meaningful to construct a therapeutic platform for targeted ammonia clearance. In this work, a biocompatible water-powered Zn micromotor is constructed as an ammonia chemotaxis platform, which can be actuated by the water splitting reaction and the self-generated Zn2+ gradient. It can propel towards NH3·H2O source through the formation of complex ions [Zn(NH3)1](OH)+ and [Zn(NH3)2](OH)+, representing a generalizable chemotaxis strategy via coordination reaction. In vivo, biomimetic collective behavior allows precise navigation and reduction of the intrahepatic ammonia level, reshaping the pathological microenvironment. This mechanism, operating in a green, zero-waste manner, facilitates integration of these micromotors into the domain of biological regulation. Such environment environment-adaptive platform is favorable for targeted treatment of hepatic fibrosis and hepatic encephalopathy caused by hyperammonemia, which is expected to provide inspiration for future personalized and precision medicine.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
The Role of Sarcopenia and Frailty in Hepatic Encephalopathy Management.Clin Liver Dis. 2015 Aug;19(3):507-28. doi: 10.1016/j.cld.2015.04.003. Clin Liver Dis. 2015. PMID: 26195205 Review.
-
Ammonia levels and the severity of hepatic encephalopathy.J Coll Physicians Surg Pak. 2014 Mar;24(3):160-3. J Coll Physicians Surg Pak. 2014. PMID: 24613109
-
[Prevention and treatment of hepatic encephalopathy].Zh Nevrol Psikhiatr Im S S Korsakova. 2017;117(10):144-147. doi: 10.17116/jnevro2017117101144-147. Zh Nevrol Psikhiatr Im S S Korsakova. 2017. PMID: 29171503 Review. Russian.
-
Refining the ammonia hypothesis: a physiology-driven approach to the treatment of hepatic encephalopathy.Mayo Clin Proc. 2015 May;90(5):646-58. doi: 10.1016/j.mayocp.2015.03.003. Epub 2015 Apr 9. Mayo Clin Proc. 2015. PMID: 25865476 Review.
-
Hepatic Encephalopathy: Current Thoughts on Pathophysiology and Management.Curr Neurol Neurosci Rep. 2025 Mar 28;25(1):28. doi: 10.1007/s11910-025-01415-9. Curr Neurol Neurosci Rep. 2025. PMID: 40153081 Review.
References
-
- Häussinger, D. Hepatocyte heterogeneity in glutamine and ammonia metabolism and the role of an intercellular glutamine cycle during ureogenesis in perfused rat liver. Eur. J. Biochem.133, 269–275 (1983). - PubMed
-
- Keiding, S., Munk, O., Roelsgaard, K., Bender, D. & Bass, L. Positron emission tomography of hepatic first-pass metabolism of ammonia in pig. Eur. J. Nucl. Med. Mol.28, 1770–1775 (2001). - PubMed
-
- Wierling, C. Bridging the gap between metabolic liver processes and functional tissue structure by integrated spatiotemporal modeling applied to hepatic ammonia detoxification. J. Gastroen. Hepatol. 60, 1823–1825 (2014). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

