Chronic rejection models for vascularized composite tissue allotransplantation
- PMID: 40374749
- PMCID: PMC12081738
- DOI: 10.1038/s41598-025-01803-8
Chronic rejection models for vascularized composite tissue allotransplantation
Abstract
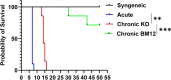
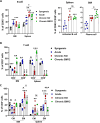
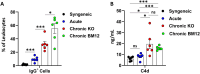
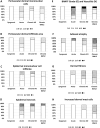
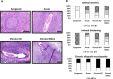
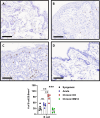
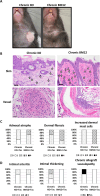
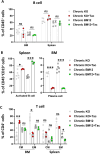
Vascularized composite tissue allotransplantation (VCA) has transformed patients' lives by enabling limb, face, abdominal wall, and penile transplants. Despite advancements in screening and immunosuppression, chronic rejection continues to limit the success of VCA. Lack of reliable preclinical models exacerbates this challenge. Here, we report on new mouse models of chronic rejection following heterotopic hind limb VCA. We employed different levels of MHC mismatch using CD8 knockout C57BL/6 mice as recipients along with BALB/c or B6 H2-Ab1bm12 mice as donors. Transient CD4 T cell depletion was induced to allow graft maturation. Evaluation included gross findings, changes in immune status changes, production of donor-specific antibodies (DSA), C4d levels, and histopathological alterations. Two chronic rejection models displayed common features of clinical chronic graft rejection, such as skin stricture, hair loss, adnexal atrophy, extensive fibrosis and mast cell infiltration without widespread necrotic changes common in acute rejection. Similar to chronic rejection patients, large populations of activated B and plasma cells were detected in the recipient's immune system as well as increased DSA and C4d production. Collectively, our models closely replicate the immunological and histopathological aspects of chronic graft rejection post-VCA, and could provide a new platform for evaluation of novel therapeutic interventions prior to clinical evaluation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Depletion of donor dendritic cells ameliorates immunogenicity of both skin and hind limb transplants.Front Immunol. 2024 May 10;15:1395945. doi: 10.3389/fimmu.2024.1395945. eCollection 2024. Front Immunol. 2024. PMID: 38799435 Free PMC article.
-
Desensitization and Prevention of Antibody-Mediated Rejection in Vascularized Composite Allotransplantation by Syngeneic Hematopoietic Stem Cell Transplantation.Transplantation. 2018 Apr;102(4):593-600. doi: 10.1097/TP.0000000000002070. Transplantation. 2018. PMID: 29298238
-
Graft vasculopathy of vascularized composite allografts in humans: a literature review and retrospective study.Transpl Int. 2019 Aug;32(8):831-838. doi: 10.1111/tri.13421. Epub 2019 Apr 2. Transpl Int. 2019. PMID: 30829423 Review.
-
The Effect of MHC Antigen Matching Between Donors and Recipients on Skin Tolerance of Vascularized Composite Allografts.Am J Transplant. 2017 Jul;17(7):1729-1741. doi: 10.1111/ajt.14189. Epub 2017 Feb 8. Am J Transplant. 2017. PMID: 28035752
-
The Significance of Vascular Alterations in Acute and Chronic Rejection for Vascularized Composite Allotransplantation.J Vasc Res. 2019;56(4):163-180. doi: 10.1159/000500958. Epub 2019 Jul 2. J Vasc Res. 2019. PMID: 31266018 Review.
References
-
- Dubernard, J. M., Owen, E. R., Lanzetta, M. & Hakim, N. What is happening with hand transplants. Lancet357, 1711–1712. 10.1016/S0140-6736(00)04846-7 (2001). - PubMed
-
- Devauchelle, B. et al. First human face allograft: Early report. Lancet368, 203–209. 10.1016/S0140-6736(06)68935-6 (2006). - PubMed
-
- Kaufman, C. L. et al. Current Status of Vascularized Composite Allotransplantation. Am. Surg.85, 631–637 (2019). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

