Temporal interference stimulation over the motor cortex enhances cortical excitability in rats
- PMID: 40374770
- PMCID: PMC12081686
- DOI: 10.1038/s41598-025-01008-z
Temporal interference stimulation over the motor cortex enhances cortical excitability in rats
Abstract
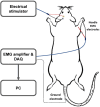
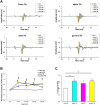
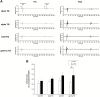
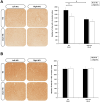
Temporal Interference Stimulation (TIS) represents a novel non-invasive brain stimulation technique that deeply targets specific brain regions using the differential beat frequency of two high-frequency stimulation pairs. This study investigated the neuromodulatory effects of TIS at different beat frequencies on cortical excitability in the rat motor cortex. Rats were randomly assigned into four groups, receiving TIS at alpha (10 Hz), beta (20 Hz), gamma (70 Hz), or sham frequencies targeting the motor cortex for 20 min under anesthesia. Cortical excitability and inhibition were evaluated by measuring motor-evoked potentials (MEPs), input-output (I/O) curves, and long-interval intracortical inhibition (LICI) before and after TIS. Additionally, immunohistochemistry was performed for neural biomarkers c-Fos and glutamic acid decarboxylase (GAD-65) to confirm targeted neural activation following TIS. We also examined glial fibrillary acidic protein (GFAP)-positive cells in the stimulated region to assess astrocyte responses associated with TIS. Alpha and gamma TIS significantly increased MEP amplitudes compared to sham stimulation. The analysis of I/O curves revealed a significant enhancement in the area under the curve (AUC) post-stimulation in the alpha and gamma TIS groups. Notably, only gamma TIS significantly reduced intracortical inhibition, indicated by an increased LICI ratio post-stimulation. Immunohistochemical analysis demonstrated a significant 35% increase in c-Fos-positive cells in the stimulated motor cortex regions after TIS compared to sham, whereas no significant changes in GAD-65-positive cells or GFAP expression were observed. These findings indicate that a single session of alpha or gamma TIS effectively modulates cortical excitability, highlighting its potential for targeted neuromodulation applications.
Keywords: Cortical excitability; Intracortical Inhibition; Motor-evoked potential; Neuromodulation; Temporal interference stimulation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interest: The authors declare no competing interests.
Figures




Similar articles
-
Neuromodulatory Responses Elicited by Intermittent versus Continuous Transcranial Focused Ultrasound Stimulation of the Motor Cortex in Rats.Int J Mol Sci. 2024 May 23;25(11):5687. doi: 10.3390/ijms25115687. Int J Mol Sci. 2024. PMID: 38891875 Free PMC article.
-
Transcranial burst electrical stimulation contributes to neuromodulatory effects in the rat motor cortex.Front Neurosci. 2023 Dec 11;17:1303014. doi: 10.3389/fnins.2023.1303014. eCollection 2023. Front Neurosci. 2023. PMID: 38146544 Free PMC article.
-
Neuromodulatory Effects of Transcranial Direct Current Stimulation on Motor Excitability in Rats.Neural Plast. 2019 Dec 17;2019:4252943. doi: 10.1155/2019/4252943. eCollection 2019. Neural Plast. 2019. PMID: 31949429 Free PMC article.
-
Advances in the application of temporal interference stimulation: a scoping review.Front Hum Neurosci. 2025 May 30;19:1536906. doi: 10.3389/fnhum.2025.1536906. eCollection 2025. Front Hum Neurosci. 2025. PMID: 40520674 Free PMC article.
-
Temporal interference stimulation for human brain: Opportunities and challenges.Innovation (Camb). 2025 Jan 15;6(4):100803. doi: 10.1016/j.xinn.2025.100803. eCollection 2025 Apr 7. Innovation (Camb). 2025. PMID: 40470330 Free PMC article. Review. No abstract available.
References
-
- Fasano, A. & Lozano, A. M. Deep brain stimulation for movement disorders: 2015 and beyond. Curr. Opin. Neurol.28(4), 423–436 (2015). - PubMed
-
- Laxton, A. W. & Lozano, A. M. Deep brain stimulation for the treatment of alzheimer disease and dementias. World Neurosurg.80(3–4), S28e1–S28e8 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

