Coupling relationship between glucose and oxygen metabolisms to serve as an imaging biomarker for Alzheimer's disease
- PMID: 40374876
- PMCID: PMC12081672
- DOI: 10.1038/s41598-025-01927-x
Coupling relationship between glucose and oxygen metabolisms to serve as an imaging biomarker for Alzheimer's disease
Abstract
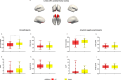
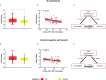
CD33 rs3865444 polymorphism is closely associated with the risk of Alzheimer's disease (AD), and CD33 is part of the sialic acid-binding Ig-superfamily of lectins (SIGLECs). Immunostaining experiments in previous studies have confirmed the expression of CD33 in human brain microglial cells, and an increase in CD33 mRNA expression in the brain microglial cells of patients with cognitive impairment has been observed. The minor allele CD33 rs3865444 (A) has a protective effect against Alzheimer's disease pathology and is associated with reduced CD33 expression and clearance of amyloid-beta plaques. The risk allele CD33 rs3865444 (C) can cause abnormal activation of microglial cells, thereby inducing neuroinflammation, accompanied by an increase in metabolic levels. We hypothesize that the CD33 rs3865444 polymorphism may affect the coupling between glucose metabolism and neuronal activity, thereby influencing individual cognitive trajectories and the progression of cognitive impairment. In this study, we included 107 patients with mild cognitive impairment, among whom the limbic-orbital frontal cortex glucose-oxygen coupling (G/O) coefficient of the CD33 rs3865444 CC group was significantly reduced. Additionally, the results of the mediation analysis showed that the glucose-oxygen coupling coefficient completely mediated the effect of the CD33 rs3865444 polymorphism on the rate of clinical dementia rating increase.
Keywords: CD33 rs3865444; Cognitive trajectory; Functional MRI (fMRI); Glycometabolism; Mild cognitive impairment; Oxygen metabolism.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical statement: This study was approved by the institutional review board (IRB) at Hangzhou Dianzi University (IRB-2020001) and the ethics committee at Beijing Hospital (2022BJYYEC-375-01). The Alzheimer’s Disease Neuroimaging Initiative has obtained informed consent from participants as research subjects, with support from the ADNI Executive Committee and representatives of the National Institute on Aging. The study investigators maintain and be responsible for determining how to use the participants’ data for future research. All links related to the participants’ identities have been removed from the data before sharing. Methods were carried out in accordance with relevant guidelines and regulations.
Figures





Similar articles
-
Association of CD33 polymorphism rs3865444 with Alzheimer's disease pathology and CD33 expression in human cerebral cortex.Neurobiol Aging. 2015 Feb;36(2):571-82. doi: 10.1016/j.neurobiolaging.2014.09.023. Epub 2014 Oct 2. Neurobiol Aging. 2015. PMID: 25448602 Free PMC article.
-
CD33 Alzheimer's risk-altering polymorphism, CD33 expression, and exon 2 splicing.J Neurosci. 2013 Aug 14;33(33):13320-5. doi: 10.1523/JNEUROSCI.1224-13.2013. J Neurosci. 2013. PMID: 23946390 Free PMC article.
-
Association of CD33 rs3865444:C˃A polymorphism with a reduced risk of late-onset Alzheimer's disease in Slovaks is limited to subjects carrying the APOE ε4 allele.Int J Immunogenet. 2020 Oct;47(5):397-405. doi: 10.1111/iji.12489. Epub 2020 Apr 24. Int J Immunogenet. 2020. PMID: 32333488
-
CD33 in Alzheimer's disease.Mol Neurobiol. 2014 Feb;49(1):529-35. doi: 10.1007/s12035-013-8536-1. Epub 2013 Aug 28. Mol Neurobiol. 2014. PMID: 23982747 Review.
-
Evaluation of CD33 as a genetic risk factor for Alzheimer's disease.Acta Neuropathol. 2019 Aug;138(2):187-199. doi: 10.1007/s00401-019-02000-4. Epub 2019 Apr 4. Acta Neuropathol. 2019. PMID: 30949760 Free PMC article. Review.
References
-
- Walsh, D. M. et al. Naturally secreted oligomers of amyloid Β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 416, 535–539 (2002). - PubMed
-
- Cirrito, J. R. et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 48, 913–922 (2005). - PubMed
-
- Wang, J-Z. & Liu, F. Microtubule-associated protein Tau in development, degeneration and protection of neurons. Prog. Neurobiol.85, 148–175 (2008). - PubMed
-
- Edison, P. et al. Microglia, amyloid, and cognition in Alzheimer’s disease: an [11 C](R) PK11195-PET and [11 C] PIB-PET study. Neurobiol. Dis.32, 412–419 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

