Genotype-specific differences in infertile men due to loss-of-function variants in M1AP or ZZS genes
- PMID: 40374915
- PMCID: PMC12162868
- DOI: 10.1038/s44321-025-00244-0
Genotype-specific differences in infertile men due to loss-of-function variants in M1AP or ZZS genes
Abstract
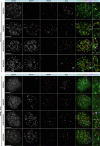
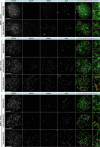
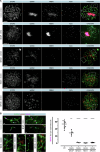
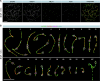
Male infertility has been linked to M1AP. In mice, M1AP interacts with the ZZS proteins SHOC1/TEX11/SPO16, promoting DNA class I crossover formation during meiosis. To determine whether M1AP and ZZS proteins are involved in human male infertility by recombination failure, we screened for biallelic/hemizygous loss-of-function (LoF) variants in the human genes to select men with presumed protein deficiency (N = 24). After in-depth characterisation of testicular phenotypes, we identified gene-specific meiotic impairments: men with ZZS deficiency shared an early meiotic arrest. Men with LoF variants in M1AP exhibited a predominant metaphase I arrest with rare haploid round or even elongated spermatids. These differences were explained by different recombination failures: deficient ZZS function led to incorrect synapsis of homologous chromosomes, unrepaired DNA double-strand breaks, and incomplete recombination. Abolished M1AP led to a reduced number of recombination intermediates and class I crossover. Medically assisted reproduction resulted in the birth of a healthy child, offering the possibility of fatherhood to men with LoF variants in M1AP. Our study establishes M1AP as an important, but non-essential, functional enhancer in meiotic recombination.
Keywords: Crossover; Infertility; M1AP; Meiosis; Recombination.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interest statement. The authors declare no competing interests.
Figures









References
-
- Arango NA, Huang TT, Fujino A, Pieretti-Vanmarcke R, Donahoe PK (2006) Expression analysis and evolutionary conservation of the mouse germ cell-specific D6Mm5e gene. Dev Dyn 235:2613–2619 - PubMed
-
- Baker SM, Plug AW, Prolla TA, Bronner CE, Harris AC, Yao X, Christie DM, Monell C, Arnheim N, Bradley A et al (1996) Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat Genet 13:336–342 - PubMed
-
- Bolcun-Filas E, Handel MA (2018) Meiosis: the chromosomal foundation of reproduction. Biol Reprod 99:112–126 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

