Effects of different anesthetic drugs on electroretinography in mice
- PMID: 40374951
- PMCID: PMC12081634
- DOI: 10.1038/s41598-025-98924-x
Effects of different anesthetic drugs on electroretinography in mice
Abstract
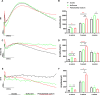
Electrophysiology (ERG) is widely used for retinal function assessment, but the effects of different anesthetics on ERG recordings, particularly in degenerated retinas, remain unclear. This study investigated the effects of different anesthetic drugs on ERG in wild-type (WT), Kv8.2 knockout (KO), and rd10 mice. Anesthetic drugs, avertin (300 mg/kg) and pentobarbital sodium (50 mg/kg) were administered intraperitoneally, isoflurane was given at 5% for induction and 1.5% for maintenance. Full-field flash electroretinography (ff-ERG) was recorded, including scotopic and photopic responses. Specifically, the amplitudes of a-wave, b-wave, oscillatory potentials (OPs), photopic negative response (PhNR), and c-wave were analyzed, respectively. Additionally, fundus imaging and optical coherence tomography (OCT) were performed to analyze retinal morphology. The three anesthetics had no obvious effect on retinal morphology. Pentobarbital sodium decreased scotopic OPs, increased scotopic and photopic b-wave amplitudes and decreased photopic a-wave amplitude in all groups of mice. Isoflurane resulted in larger scotopic OPs and photopic a-wave amplitudes in all groups, with a larger scotopic a-wave amplitude in KO mice. The PhNR amplitude was greater in WT mice anesthetized with avertin. The ERG amplitudes in mice showed significant differences among the three anesthetic conditions. Pentobarbital sodium markedly suppressed retinal OPs, suggesting it may not be suitable for assessing inner retinal function, particularly amacrine cells. Isoflurane enabled excellent recordings of various types of ERG, making it suitable for nearly all ERG recordings. Avertin might serve as a suitable alternative in the absence of isoflurane.
Keywords: Avertin; ERG; Isoflurane; Kv8.2 KO; Pentobarbital sodium; rd10.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
ERG Responses in Mice with Deletion of the Synaptic Ribbon Component RIBEYE.Invest Ophthalmol Vis Sci. 2020 May 11;61(5):37. doi: 10.1167/iovs.61.5.37. Invest Ophthalmol Vis Sci. 2020. PMID: 32437548 Free PMC article.
-
Effects of common anesthetics on eye movement and electroretinogram.Doc Ophthalmol. 2011 Jun;122(3):163-76. doi: 10.1007/s10633-011-9271-4. Epub 2011 Apr 26. Doc Ophthalmol. 2011. PMID: 21519880 Free PMC article.
-
Inter-ocular and inter-session reliability of the electroretinogram photopic negative response (PhNR) in non-human primates.Exp Eye Res. 2004 Jan;78(1):83-93. doi: 10.1016/j.exer.2003.09.013. Exp Eye Res. 2004. PMID: 14667830
-
Comparing three different modes of electroretinography in experimental glaucoma: diagnostic performance and correlation to structure.Doc Ophthalmol. 2017 Apr;134(2):111-128. doi: 10.1007/s10633-017-9578-x. Epub 2017 Feb 27. Doc Ophthalmol. 2017. PMID: 28243926 Free PMC article.
-
[Animal models of human retinal and optic nerve diseases analysed using electroretinography].Nippon Ganka Gakkai Zasshi. 2010 Mar;114(3):248-78, discussion 279. Nippon Ganka Gakkai Zasshi. 2010. PMID: 20387538 Review. Japanese.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

