Evaluation of Data Quality and Utility of the Japan Drug Information Institute in Pregnancy (JDIIP) Consultation Case Database for Pregnancy Pharmacovigilance
- PMID: 40374964
- PMCID: PMC12334455
- DOI: 10.1007/s40264-025-01554-5
Evaluation of Data Quality and Utility of the Japan Drug Information Institute in Pregnancy (JDIIP) Consultation Case Database for Pregnancy Pharmacovigilance
Abstract
Introduction: Ensuring medication safety during pregnancy is crucial for protecting maternal and fetal health. However, fragmented data sources and the lack of comprehensive databases present substantial barriers to effective pharmacovigilance. The Japan Drug Information Institute in Pregnancy (JDIIP) database, which contains data on drug treatment counseling for pregnant women, is expected to help address the lack of comprehensive databases for pregnancy pharmacovigilance (PregPV).
Objective: We evaluated the quality and utility of the JDIIP database for PregPV activities, particularly its ability to consolidate and utilize drug-exposure data among pregnant women in Japan.
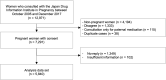
Methods: To assess the quality and utility of the JDIIP database for PregPV, we examined its alignment with 48 core data elements (CDEs) considered critical for PregPV, as recently proposed by a European Union consortium through the ConcePTION Project. We performed a detailed mapping of each CDE definition-including maternal lifestyle factors, drug exposure, and pregnancy outcomes-against the corresponding data elements captured in the JDIIP database.
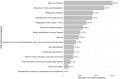
Results: The JDIIP database either directly collected or could derive 38 of the 48 specific items (79%) recommended by the ConcePTION Project. At the category level, the JDIIP database aligned closely with the CDE requirements for database management details, pregnancy details, maternal medical history, pregnancy medication exposure, live/stillborn birth outcomes, and malformation details, achieving coverage of over 80% of the necessary variables in each category. Some categories, such as maternal medical conditions arising during pregnancy and infant complications within the first year of life, showed less alignment, with coverage rates below 50%. Although the JDIIP database provides comprehensive coverage of critical pharmacovigilance elements, data collection for specific variables and categories that better align with the CDE framework can be enhanced to improve alignment with the CDE framework and strengthen pharmacovigilance capabilities.
Conclusions: Our findings highlight the potential of the JDIIP database as a valuable resource for advancing PregPV research. Although the collection of certain maternal and infant data elements could be improved, the substantial alignment of the database with established CDEs positions it as a promising tool for advancing PregPV initiatives in Japan.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This research was supported by a grant from the Institute of Statistical Mathematics. Conflicts of Interest: Shinichi Matsuda is employed by Chugai Pharmaceutical Co., Ltd. Manabu Akazawa has received consulting fees and honoraria from Astellas Pharma Inc., Janssen Pharmaceutical K.K., Shionogi & Co., Ltd, GSK, and Mitsubishi Tanabe Pharma Corporation. Mihoko Ota was employed by Takeda Pharmaceutical Co., Ltd before the submission of this work. Hiroaki Oka is employed by Shionogi & Co., Ltd. Naoki Nitani is employed by CMIC HealthCare Institute Co., Ltd. Naho Yakuwa, Mikako Goto, Kunihiko Takahashi, Tatsuhiko Anzai, Sachi Koinuma, Izumi Fujioka, Yoriko Miura, Tomiko Tawaragi, and Atsuko Murashima have no relevant financial or non-financial interests to disclose. Ethics Approval: This study received ethical approval from the ethics committee of the National Center for Child Health and Development under ethical review number 2020–005. Consent to Participate: Written informed consent was obtained from all individual participants involved in the study. Consent for Publication: Not applicable. Availability of Data and Material: The data supporting this study’s findings are not openly available due to sensitivity reasons but are available from the corresponding author upon reasonable request. Code Availability: Not applicable. Author Contributions: All authors contributed to the study conceptualization and design. Naho Yakuwa, Mikako Goto, Sachi Koinuma, Izumi Fujioka, Yoriko Miura, and Atsuko Murashima contributed to the data acquisition. Shinichi Matsuda, Naho Yakuwa, Mikako Goto, Manabu Akazawa, Kunihiko Takahashi, Tatsuhiko Anzai, and Tomiko Tawaragi performed the data analysis. All authors contributed to the data interpretation, drafting, and revision of the manuscript. All authors have read and approved the final version of the manuscript.
Figures
Similar articles
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Telephone support for women during pregnancy and the first six weeks postpartum.Cochrane Database Syst Rev. 2013 Jul 18;2013(7):CD009338. doi: 10.1002/14651858.CD009338.pub2. Cochrane Database Syst Rev. 2013. PMID: 23881662 Free PMC article.
-
Interventions to improve safe and effective medicines use by consumers: an overview of systematic reviews.Cochrane Database Syst Rev. 2014 Apr 29;2014(4):CD007768. doi: 10.1002/14651858.CD007768.pub3. Cochrane Database Syst Rev. 2014. PMID: 24777444 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Multiple-micronutrient supplementation for women during pregnancy.Cochrane Database Syst Rev. 2017 Apr 13;4(4):CD004905. doi: 10.1002/14651858.CD004905.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2019 Mar 14;3:CD004905. doi: 10.1002/14651858.CD004905.pub6. PMID: 28407219 Free PMC article. Updated.
References
-
- Irvine L, Flynn RW, Libby G, Crombie IK, Evans JM. Drugs dispensed in primary care during pregnancy: a record-linkage analysis in Tayside, Scotland. Drug Saf. 2010;33:593–604. 10.2165/11532330-000000000-00000. - PubMed
-
- Gerbier E, Panchaud A. Specialty grand challenge editorial innovative approaches for pharmacoepidemiologic research in pregnancy: shifting the paradigm of thalidomide’s impact on pregnant women. Front Drug Saf Regul. 2023;31(3):1187070. 10.3389/fdsfr.2023.1187070.
-
- Ward RM. Difficulties in the study of adverse fetal and neonatal effects of drug therapy during pregnancy. Semin Perinatol. 2001;25:191–5. 10.1053/sper.2001.24567. - PubMed
-
- Werler MM, Mitchell AA, Shapiro S. The relation of aspirin use during the first trimester of pregnancy to congenital cardiac defects. N Engl J Med. 1989;321:1639–42. 10.1056/NEJM198912143212404. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

