Proinflammatory cytokine-induced alpha cell impairment in human islet microtissues is partially restored by dual incretin receptor agonism
- PMID: 40374968
- PMCID: PMC12176928
- DOI: 10.1007/s00125-025-06425-3
Proinflammatory cytokine-induced alpha cell impairment in human islet microtissues is partially restored by dual incretin receptor agonism
Abstract
Aims/hypothesis: In type 1 diabetes, the counterregulatory glucagon response to low plasma glucose is impaired. The resulting increased risk of hypoglycaemia necessitates novel strategies to ameliorate alpha cell impairment. Here, we aimed to establish an in vitro type 1 diabetes-like model of alpha cell impairment using standardised reaggregated human islet microtissues (MTs) exposed to proinflammatory cytokines. Additionally, we investigated the therapeutic potential of incretin receptor agonists in improving alpha cell responses to low glucose.
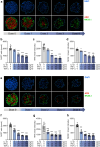
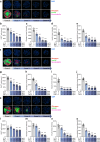
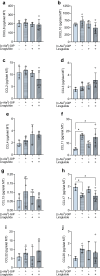
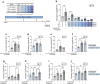
Methods: Human islet MTs were exposed to proinflammatory cytokines (IL-1β, IFN-γ and TNF-α) for 1 day (short-term) and 6 days (long-term). Alpha cell function was assessed by sequential glucose-dependent secretion assays at 2.8 and 16.7 mmol/l glucose, followed by glucagon measurements. Additional evaluations included ATP content, caspase-3/7 activity, chemokine secretion and content of islet transcription factors (aristaless-related homeobox [ARX] and NK6 homeobox 1 [NKX6.1]) and hormones. The effects of incretin receptor agonist treatment (glucose-dependent insulinotropic polypeptide [GIP] analogue [D-Ala2]-GIP with or without the glucagon-like peptide 1 [GLP-1] receptor agonist liraglutide) alongside or after cytokine exposure were also investigated, focusing on low-glucose-dependent glucagon secretion.
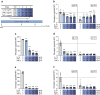
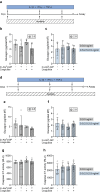
Results: Short-term cytokine exposure increased glucagon secretion at both 2.8 and 16.7 mmol/l glucose in islet MTs. In contrast, long-term cytokine exposure caused dose-dependent suppression of glucagon secretion at 2.8 mmol/l glucose, resembling a type 1 diabetes phenotype. Long-term cytokine exposure also diminished insulin and somatostatin secretion, reduced ATP content, increased caspase 3/7 activity and decreased islet content of ARX, NKX6.1, glucagon and insulin. Despite cytokine-induced impairment, alpha cells partially retained secretory capacity to L-arginine stimulation. Treatment with incretin receptor agonists during long-term cytokine exposure did not prevent alpha cell impairment. However, acute treatment with [D-Ala2]-GIP with or without liraglutide, or with the single-molecule dual agonist tirzepatide, after cytokine exposure partially restored glucagon secretion at low glucose.
Conclusions/interpretation: Long-term cytokine exposure of human islet MTs created a type 1 diabetes-like phenotype with impaired low-glucose-induced glucagon secretion. This cytokine-induced alpha cell impairment was partially restored by [D-Ala2]-GIP with or without liraglutide, or by tirzepatide.
Keywords: GIP; GLP-1; Glucagon secretion; Human model; Incretins; Liraglutide; Pancreatic alpha cells; Proinflammatory cytokines; Type 1 diabetes.
© 2025. The Author(s).
Conflict of interest statement
Acknowledgements: The authors gratefully acknowledge organ donors and the next of kin of organ donors, without whom this research would not be possible. The authors thank R. Gerwig from Steno Diabetes Center Copenhagen and L. B. Albæk from the University of Copenhagen for the technical support with the chemokine and somatostatin measurements, respectively. Data availability: The datasets generated and analysed during the study are available from the corresponding author upon reasonable request. Funding: Open access funding provided by Copenhagen University. This paper represents independent research supported by funding from The Leona M. and Harry B. Helmsley Charitable Trust (grant no. 1912-03551) and the Poul and Erna Sehested-Hansen Foundation. No funders were involved in the study design, data collection, analysis, interpretation, writing or decision to submit the article for publication. Authors’ relationships and activities: CR, ACT, SJ and BY are employees of InSphero. BH is a co-founder of Bainan Biotech. JJH has served on scientific advisory panels for and/or has received speaker honoraria from Novo Nordisk and MSD/Merck, is co-founder and board member of Antag Therapeutics, and is co-founder of Bainan Biotech. FKK has served on scientific advisory panels and/or been part of speaker’s bureaus for, served as a consultant to, and/or received research support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Sanofi, ShouTi, Zealand Pharma and Zucara. FKK is a co-founder of and minority shareholder in Antag Therapeutics, owns stocks in Eli Lilly, Novo Nordisk and Zealand Pharma, and has been employed by Novo Nordisk since 1 December 2023. JS owns stocks in Novo Nordisk A/S. The authors declare that there are no other relationships or activities that might bias, or be perceived to bias, their work. Contribution statement: KH, FKK and JS conceptualised the study. KH, BY and JS designed the experiments. KH, CR, SJ, ACT, BH, JJH and BY contributed to the data acquisition and analysis. KH wrote the initial manuscript draft and JS provided comments. All authors provided critical scientific input to the manuscript and approved the final version. JS is the guarantor of this work.
Figures

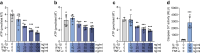

Similar articles
-
Evaluating glucose-dependent insulinotropic polypeptide and glucagon as key regulators of insulin secretion in the pancreatic islet.Am J Physiol Endocrinol Metab. 2024 Jul 1;327(1):E103-E110. doi: 10.1152/ajpendo.00360.2023. Epub 2024 May 22. Am J Physiol Endocrinol Metab. 2024. PMID: 38775725 Free PMC article. Review.
-
The incretin co-agonist tirzepatide requires GIPR for hormone secretion from human islets.Nat Metab. 2023 Jun;5(6):945-954. doi: 10.1038/s42255-023-00811-0. Epub 2023 Jun 5. Nat Metab. 2023. PMID: 37277609 Free PMC article.
-
Small molecule glucagon release inhibitors with activity in human islets.Front Endocrinol (Lausanne). 2023 Apr 19;14:1114799. doi: 10.3389/fendo.2023.1114799. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37152965 Free PMC article.
-
TMEM55A-mediated PI5P signalling regulates alpha cell actin depolymerisation and glucagon secretion.Diabetologia. 2025 Jul;68(7):1509-1523. doi: 10.1007/s00125-025-06411-9. Epub 2025 Mar 26. Diabetologia. 2025. PMID: 40140059 Free PMC article.
-
Evolving incretin-based therapies in Japan: optimizing treatment strategies for diverse clinical and socioeconomical profiles in type 2 diabetes.Diabetol Int. 2025 May 15;16(3):457-468. doi: 10.1007/s13340-025-00818-w. eCollection 2025 Jul. Diabetol Int. 2025. PMID: 40607140 Free PMC article. Review.
References
-
- Gerich JE, Langlois M, Noacco C, Karam JH, Forsham PH (1973) Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science 182(4108):171–173. 10.1126/SCIENCE.182.4108.171 - PubMed
-
- Cryer PE (2014) Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes 63:2188–2195. 10.2337/db14-0059 - PubMed
-
- Wendt A, Eliasson L (2020) Pancreatic α-cells – the unsung heroes in islet function. Semin Cell Dev Biol 103:41–50. 10.1016/J.SEMCDB.2020.01.006 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

