SEC24D depletion induces osteogenic differentiation deficiency by inactivating the ATF6/TGF-β/Runx2 regulatory loop
- PMID: 40374976
- PMCID: PMC12081754
- DOI: 10.1038/s42003-025-08175-9
SEC24D depletion induces osteogenic differentiation deficiency by inactivating the ATF6/TGF-β/Runx2 regulatory loop
Abstract
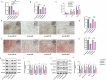
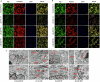
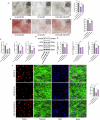
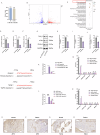
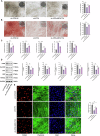
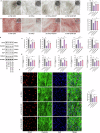
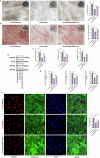
Protein coat complexes strongly influence intracellular cargo trafficking. Coatopathies represent a wide range of genetic conditions caused by mutations in protein coat components. The SEC24D gene, which encodes a Sec24 isoform that constitutes a cargo-specific capturer in the COPII coat, is responsible for a rare type of autosomal recessive osteogenesis imperfecta. We report an OI patient. Clinical and imaging findings suggested that the patient had OI. Genetic detection by whole-exome sequencing (WES) identified a compound heterozygous SEC24D variants, including c.2609_2610delGA (p. R870fs*10) and c.938G>A (p. R313H). In silico analysis suggested that the missense R313H mutation most likely affects protein stability and secondary structure. In vitro studies showed that knockdown or mutation of SEC24D affected the osteogenic differentiation of mesenchymal stem cells (MSCs) and inducted ER stress. Transcriptomic sequencing suggested that the TGF-β pathway mediated the destructive effect of SEC24D depletion on osteogenic differentiation. Further experiments confirmed that ATF6 participated in regulating the TGF-β pathway and osteogenic biomarkers by SEC24D. This study identified a SEC24D variation causing OI, which expanded the mutation spectrum of this gene. Further studies on the mechanism of action showed that SEC24D defects may induce osteogenic differentiation deficiency by inactivating the ATF6/TGF-β/Runx2 regulatory loop.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This study was approved by the Ethics Committee of Shijiazhuang Obstetrics and Gynecology Hospital (approval No. 20240057), and written informed consent was obtained from all participants.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

