European Reference Network (ERN) ReCONNET methodology for the cross-cultural adaptation of instruments for research and care in the context of rare connective tissue diseases (CROSSADAPT)
- PMID: 40375068
- PMCID: PMC12080175
- DOI: 10.1186/s13023-025-03674-8
European Reference Network (ERN) ReCONNET methodology for the cross-cultural adaptation of instruments for research and care in the context of rare connective tissue diseases (CROSSADAPT)
Abstract
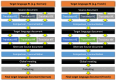
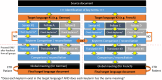
The traditional process of intercultural adaptation, while suitable for one or a few target languages, is not optimal for developing instruments for rare connective tissue diseases (CTDs) in multiple languages simultaneously. The European Reference Network ReCONNET presents the protocol for a novel methodology for cross-cultural adaptation of instruments for research and care in the context of rare CTDs (ReCONNET-CROSSADAPT). It is initiated by the identification of 'key-terms' that are crucial for maintaining the original meaning of the source document. Each language group, led by a senior member and two collaborators, independently assesses the existence and equivalence of these key terms in target languages. Reconciliation meetings are held to establish agreed-upon terms for consistent usage across translations when difficulties arise with key-terms. Subsequently, each language group translates the source document, followed by a reconciliation meeting involving one CTD patient in each group. The purpose of this meeting is to address potential discrepancies among translations, ensuring a comprehensive assessment from a linguistic, cultural and patient perspective. Collective feedback and consensus-based decision-making guide the resolution process. This methodology eliminates the need for backward translation, optimizing time and cost utilization. This new ReCONNET-CROSSADAPT methodology ensures linguistic accuracy, cultural relevance, and contextual appropriateness for the cross-cultural adaptation of instruments for research and care in the context of rare CTDs.
Keywords: Cross-cultural adaptation; Orphan diseases; Rare diseases; Reference networks; Rheumatology.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by comité d'éthique des Facultés de Médecine, d'Odontologie, de Pharmacie, des Ecoles d’Infirmières, de Kinésithérapie, de Maïeutique et des Hôpitaux de Strasbourg (CE-2024-15). All participants consented to participation. Consent for publication: All authors consent to publication. Competing interests: The authors report no disclosure related to this manuscript.
Figures
References
-
- Talarico R, Aguilera S, Alexander T, Amoura Z, Andersen J, Arnaud L, et al. The added value of a European Reference Network on rare and complex connective tissue and musculoskeletal diseases: insights after the first 5 years of the ERN ReCONNET. Clin Exp Rheumatol. 2022;40(5 suppl. 134):3–11. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical