Machine learning for predicting all-cause mortality of metabolic dysfunction-associated fatty liver disease: a longitudinal study based on NHANES
- PMID: 40375096
- PMCID: PMC12082853
- DOI: 10.1186/s12876-025-03946-4
Machine learning for predicting all-cause mortality of metabolic dysfunction-associated fatty liver disease: a longitudinal study based on NHANES
Abstract
Background: The mortality burden of metabolic dysfunction-associated fatty liver disease (MAFLD) is rising, making it crucial to predict mortality and identify the factors influencing it. While advanced machine learning algorithms are gaining recognition as effective tools for clinical prediction, their ability to predict all-cause mortality of MAFLD individuals remains uncertain. This study aimed to develop different machine learning models to predict all-cause mortality of MAFLD individuals, compare the predictive performance of these models, and identify the risk factors contributing all-cause mortality, which is crucial for management of MAFLD individuals.
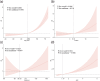
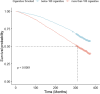
Methods: We included 3921 MAFLD individuals in NHANES III. After a median follow-up time of 310 months, 1815 (46.3%) deaths were recorded. The data (demographic, behavioral factors and laboratory indicators) were utilized to construct machine learning models (Coxnet, RSF, GBS) after feature selection. Time-dependent AUC, time-dependent brier and C-index were then evaluated the performance of models. We identified the top five factors that contributed significantly to all-cause mortality and further explore the association with all-cause mortality using RCS and Kaplan-Meier survival curves.
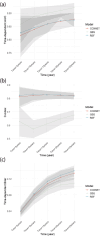
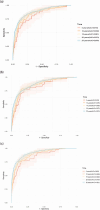
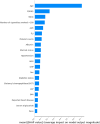
Results: Coxnet showed the best performance in short-term and long-term predictions with time-dependent AUC of 0.82 at 5 years and 0.88 at 25 years. Age, FORNS, waist circumstance, AAR, FLI were associated positively with all-cause mortality. Compared to the individuals who smoked more than 100 cigarettes, those below 100 had better survival outcome (P < 0.0001).
Conclusions: Machine learning has a promising application in predicting all-cause mortality in MAFLD individuals. Combined the results of interpretable machine learning and association analyses, we found risk factors which contributing to the all-cause mortality. These findings provide insights for community health practitioners to intervene in modifiable risk factors, thereby improving the survival and quality of life of MAFLD individuals.
Keywords: All-cause mortality; Fatty liver; Machine learning; Metabolic dysfunction-associated fatty liver disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests. Supplementary Information: Supplementary Material 1.
Figures
References
-
- Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–9. - PubMed
-
- Pennisi G, Infantino G, Celsa C, Di Maria G, Enea M, Vaccaro M, et al. Clinical outcomes of MAFLD versus NAFLD: a meta-analysis of observational studies. Liver Int. 2024;44(11):2939–49. - PubMed
-
- Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999-2014.e1. - PubMed
-
- Kapoor N, Kalra S. Metabolic-associated fatty liver disease and diabetes: a double whammy. Endocrinol Metab Clin North Am. 2023;52(3):469–84. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

