Endogenous Galectin-8 protects against Th17 infiltration and fibrosis following acute kidney injury
- PMID: 40375122
- PMCID: PMC12083165
- DOI: 10.1186/s10020-025-01245-y
Endogenous Galectin-8 protects against Th17 infiltration and fibrosis following acute kidney injury
Abstract
Background: Acute kidney injury (AKI) is a serious clinical condition characterized by a rapid decline in renal function, often progressing to chronic kidney disease (CKD) and fibrosis. The endogenous mechanisms influencing kidney injury resolution or maladaptive repair remain poorly understood. Galectin-8 (Gal-8), a tandem-repeat β-galactoside-binding lectin, plays a role in epithelial cell proliferation, epithelial-mesenchymal transition, and immune regulation, all of which are critical in AKI outcomes. While exogenous Gal-8 administration has shown renoprotective effects, its endogenous role in kidney injury progression and resolution remains unclear.
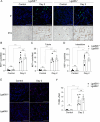
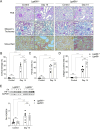
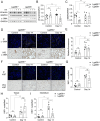
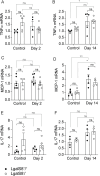
Methods: To investigate the endogenous role of Gal-8 in AKI, we compared the responses of Gal-8 knockout (Gal-8-KO; Lgals8-/- bearing a β-gal cassette under the Lgals8 gene promoter) and wild-type (Lgals8+/+) mice in a nephrotoxic folic acid (FA)-induced AKI model. Renal Gal-8 expression was assessed by β-galactosidase staining, lectin-marker colocalization, and RT-qPCR. Renal function, structure, and immune responses were evaluated at the acute (day 2) and fibrotic (day 14) phases of injury. Plasma creatinine levels were measured to assess renal function, while histological analyses evaluated tubular damage, renal inflammation, and extracellular matrix deposition. Flow cytometry was performed to characterize the immune response, focusing on pro-inflammatory T cells.
Results: Galectin-8 was predominantly expressed in the renal cortex, localizing to tubules, glomeruli, and blood vessels, with its levels decreasing by half following AKI. Both Lgals8+/+ and Lgals8-/- mice exhibited similar renal function and structure impairments during the acute phase, though Lgals8+/+ mice showed slightly worse damage. By the fibrotic phase, Lgals8-/- mice exhibited more pronounced cortical damage and fibrosis, characterized by increased type I and III collagen deposition and enhanced Th17 cell infiltration, while myofibroblast activation remained comparable to that of Lgals8+/+ mice.
Conclusions: Endogenous Gal-8 does not significantly protect the kidney during the acute phase and is dispensable for cell proliferation and death in response to AKI. However, it is crucial in preventing maladaptive repair by regulating extracellular matrix homeostasis and mitigating fibrosis. Additionally, Gal-8 contributes to inflammation resolution by limiting persistent immune cell infiltration, particularly IL-17-secreting cells.
Keywords: AKI; Acute kidney injury; CDK; Chronic kidney disease; Fibrosis; Galectin-8; Inflammation and Th17.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study has been approved by the Ethical Committee of Universidad San Sebastián (Protocol number 01–2021-10). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Abu El-Asrar AM, Missotten L, Geboes K. Expression of myofibroblast activation molecules in proliferative vitreoretinopathy epiretinal membranes. Acta Ophthalmol. 2011;89:e115-121. - PubMed
-
- Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens. 2004;13:1–7. - PubMed
-
- Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887-899. - PubMed
MeSH terms
Substances
Grants and funding
- FB210008/ANID Centro Científico Tecnológico de Excelencia Ciencia & Vida Basal Project
- FB210008/ANID Centro Científico Tecnológico de Excelencia Ciencia & Vida Basal Project
- FB210008/ANID Centro Científico Tecnológico de Excelencia Ciencia & Vida Basal Project
- FB210008/ANID Centro Científico Tecnológico de Excelencia Ciencia & Vida Basal Project
- 1210013/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1221796/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1231909/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1221067/Fondo Nacional de Desarrollo Científico y Tecnológico
- 1211829/Fondo Nacional de Desarrollo Científico y Tecnológico
- USS-FIN-24-CNGI-66./Vicerrectoría de Investigación y Doctorados de la Universidad San Sebastián
LinkOut - more resources
Full Text Sources
Research Materials

