Taurultam shows antiviral activity against SARS-CoV-2 and influenza virus
- PMID: 40375181
- PMCID: PMC12080262
- DOI: 10.1186/s12866-025-03847-2
Taurultam shows antiviral activity against SARS-CoV-2 and influenza virus
Abstract
Background: SARS-CoV-2 and influenza virus are highly contagious respiratory viruses that continuously pose major threats to human and public health. The high frequency of viral mutations led to the emergence of resistant isolates and caused virus epidemics repeatedly, emphasizing the urgent need to develop new antivirals. Taurultam is a metabolite of taurolidine. Moreover, taurolidine has been shown to have potent antiviral activities against multiple viruses and to have antiviral effects through its metabolites.
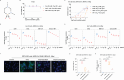
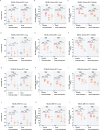
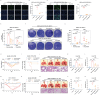
Results: In this study, we sought to determine the antiviral activities of taurultam against SARS-CoV-2 and influenza virus in Vero-E6, Huh7, 293T-ACE2, and MDCK cell lines and mouse infection models. The results showed that taurultam exhibited potent antiviral activity against multiple SARS-CoV-2 variants, influenza A (H1N1, H3N2) virus and influenza B virus, in vitro. Moreover, in influenza A (H1N1) virus, influenza B virus and SARS-CoV-2 infection models, taurultam significantly reduced viral loads, increased survival, improved mouse body weight and lung injury. Surprisingly, taurultam treatment not only inhibited the influenza A virus and SARS-CoV-2, but also benefited for therapy of mixed infection of these two viruses in vitro, demonstrating the great antiviral potential of taurultam for the treatment of SARS-CoV-2 and influenza virus infections.
Conclusions: Together, our findings identify taurultam as a new candidate for the treatment of SARS-CoV-2 and influenza virus infections, especially virus-induced lung pathology.
Clinical trial number: Not applicable.
Keywords: Antiviral; Influenza virus; Lung injury; SARS-CoV-2; Taurolidine metabolite; Taurultam.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Animal ethics approval and biosafety statement: Animal experiments were conducted following the protocols of the Changchun Veterinary Research Institute, Chinese Academy of Agricultural Sciences (approval number: IACUC of AMMS-11-2022-036). The mice were anaesthetized via inhalation of 2.0% isoflurane, infected with nasal viral drops, and then naturally awakened. The endpoint of observation was defined as either natural death or a weight less than 75% of the original weight. The mice were euthanized once their weight was lower than 75% of the initial body weight via the inhalant euthanasia agent carbon dioxide. Additionally, all influenza virus infection experiments were conducted in a biosafety level 2 laboratory, and all SARS-CoV-2 infection experiments were conducted in a biosafety level 3 laboratory according to biosafety regulations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Synergistic effects of Lianhuaqingwen in combination with Oseltamivir and Baloxavir against seasonal influenza virus: In vitro and in vivo assessment.J Ethnopharmacol. 2025 Feb 10;338(Pt 2):119091. doi: 10.1016/j.jep.2024.119091. Epub 2024 Nov 9. J Ethnopharmacol. 2025. PMID: 39528119
-
In vitro antiviral activities of thymol and Limonin against influenza a viruses and SARS-CoV-2.Sci Rep. 2025 Jul 2;15(1):22587. doi: 10.1038/s41598-025-06967-x. Sci Rep. 2025. PMID: 40596436 Free PMC article.
-
Biocompatible Iron Oxide Nanoparticles Display Antiviral Activity Against Two Different Respiratory Viruses in Mice.Int J Nanomedicine. 2024 Dec 21;19:13763-13788. doi: 10.2147/IJN.S475323. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39723174 Free PMC article.
-
Identification and Characterization of Novel Compounds with Broad-Spectrum Antiviral Activity against Influenza A and B Viruses.J Virol. 2020 Mar 17;94(7):e02149-19. doi: 10.1128/JVI.02149-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31941776 Free PMC article.
-
Shutting the gate before the horse has bolted: is it time for a conversation about SARS-CoV-2 and antiviral drug resistance?J Antimicrob Chemother. 2021 Aug 12;76(9):2230-2233. doi: 10.1093/jac/dkab189. J Antimicrob Chemother. 2021. PMID: 34142123 Free PMC article. Review.
References
-
- Zhang W, et al. Frequency and distribution of H1N1 influenza A viruses with oseltamivir-resistant mutations worldwide before and after the 2009 pandemic. J Med Virol. 2022;94(9):4406–16. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

