Tyrosinase in melanoma inhibits anti-tumor activity of PD-1 deficient T cells
- PMID: 40375241
- PMCID: PMC12083179
- DOI: 10.1186/s12915-025-02237-4
Tyrosinase in melanoma inhibits anti-tumor activity of PD-1 deficient T cells
Erratum in
-
Author Correction: Tyrosinase in melanoma inhibits anti-tumor activity of PD-1 deficient T cells.BMC Biol. 2025 Jun 15;23(1):165. doi: 10.1186/s12915-025-02285-w. BMC Biol. 2025. PMID: 40518525 Free PMC article. No abstract available.
Abstract
Background: Melanoma is one of the most commonly diagnosed malignancies and serves as a model for studying immunotherapy. The B16 melanoma model, resembling human cold tumors that lack T cell infiltration and show minimal response to PD-1 blockade, is widely used for studying melanoma and its resistance to immunotherapy. Therefore, understanding the molecular basis that prevents T cell-mediated anti-tumor activity in B16 melanoma is of great significance.
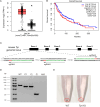
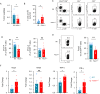
Results: In this study, we generated tyrosinase knockout B16 melanoma cells using CRISPR/Cas9 and discovered that tyrosinase in melanoma significantly inhibits the anti-tumor activity of T cells. Tyrosinase deficiency leads to a 3.80-fold increase in T-cell infiltration and enhances T-cell activation within the tumor. Single-cell RNA sequencing reveals an altered cold tumor immunophenotype in tyrosinase-deficient B16 melanoma. In wild-type mice, T cells in tyrosinase-deficient tumors express elevated levels of PD-1 and Foxp3. However, strikingly, in PD-1 deficient mice, the loss of tyrosinase in B16 melanoma unleashes the anti-tumor activity of PD-1 deficient T cells. This enhanced anti-tumor activity is explained by significantly increased tumor T cell infiltration accompanied by reduced frequencies of regulatory T cells in PD-1 knockout mice.
Conclusions: These findings suggest that targeting tyrosinase could convert cold tumors into an immune-responsive state in vivo using murine models. Inhibiting tyrosinase could enhance the effectiveness of PD-1 blockade, offering a new approach for melanoma patients who fail in current PD-1 inhibitor treatment.
Keywords: Melanoma; PD-1; Tumor infiltrating T cells; Tyrosinase.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All institutional and national guidelines for the care and use of laboratory animals were followed. The procedures were conducted according to the Standard of Laboratory Animals—General Code of Animal Welfare (GB/T 42011 − 2022) and approved by the animal care committee at Xinxiang Medical University. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Pelayo BA, Fu YM, Meadows GG. Decreased tissue plasminogen activator and increased plasminogen activator inhibitors and increased activator protein-1 and specific promoter 1 are associated with inhibition of invasion in human A375 melanoma deprived of tyrosine and phenylalanine. Int J Oncol. 2001;18(4):877–83. - PubMed
-
- Fu YM, Yu ZX, Pelayo BA, Ferrans VJ, Meadows GG. Focal adhesion kinase-dependent apoptosis of melanoma induced by tyrosine and phenylalanine deficiency. Cancer Res. 1999;59(3):758–65. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

