A validated multivariable machine learning model to predict cardio-kidney risk in diabetic kidney disease
- PMID: 40375260
- PMCID: PMC12082972
- DOI: 10.1186/s12933-025-02779-5
A validated multivariable machine learning model to predict cardio-kidney risk in diabetic kidney disease
Abstract
Background: Individuals with diabetic kidney disease (DKD) often suffer cardiac and kidney events. We sought to develop an accurate means by which to stratify risk in DKD.
Methods: Clinical variables and biomarkers were evaluated for their ability to predict the adjudicated primary composite endpoint of CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) by 3 years. Using machine learning techniques, a parsimonious risk algorithm was developed.
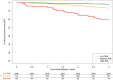
Results: The final model included age, body-mass index, systolic blood pressure, and concentrations of N-terminal pro-B type natriuretic peptide, high sensitivity cardiac troponin T, insulin-like growth factor binding protein-7 and growth differentiation factor-15. The model had an in-sample C-statistic of 0.80 (95% CI = 0.77-0.83; P < 0.001). Dividing results into low, medium and high risk categories, for each increase in level the hazard ratio increased by 3.43 (95% CI = 2.72-4.32; P < 0.001). Low risk scores had negative predictive value of 94%, while high risk scores had positive predictive value of 58%. Higher values were associated with shorter time to event (log rank P < 0.001). Rising values at 1 year predicted higher risk for subsequent DKD events. Canagliflozin treatment reduced score results by 1 year with consistent event reduction across risk levels. Accuracy of the risk model was validated in separate cohorts from CREDENCE and the generally lower risk Canagliflozin Cardiovascular Assessment Study.
Conclusions: We describe a validated risk algorithm that accurately predicts cardio-kidney outcomes across a broad range of baseline risk.
Trial registration: CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; NCT02065791) and CANVAS (Canagliflozin Cardiovascular Assessment Study; NCT01032629/NCT01989754).
Keywords: Canagliflozin; Diabetes mellitus; Diabetic kidney disease; Prognosis; Risk prediction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All study procedures for CREDENCE and CANVAS and subsequent analyses were approved by local ethics committees. Written informed consent was obtained for participation in both studies, including analyses of biomarkers. Competing interests: Dr. Januzzi reports equity holdings in Imbria Pharma, Jana Care, and Fibrosys, current/recent grant support from Abbott, Applied Therapeutics, AstraZeneca, BMS, Novartis Pharmaceuticals, consulting income from Abbott Diagnostics, Beckman-Coulter, Jana Care, Janssen, Novartis, Prevencio, Quidel, and Roche Diagnostics and serves on clinical endpoint committees/data safety monitoring boards for Abbott, AbbVie, Amgen, CVRx, Medtronic, Pfizer, and Roche Diagnostics; Dr. Sattar has consulted for and/or received speaker honoraria from Abbott Laboratories, AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Janssen, Menarini-Ricerche, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Sanofi; he has received grant support paid to his university from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics outside the submitted work; Dr. Vaduganathan has received research grant support, served on advisory boards, or had speaker engagements with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, BMS, Boehringer Ingelheim, Chiesi, Cytokinetics, Fresenius Medical Care, Idorsia Pharmaceuticals, Lexicon Pharmaceuticals, Merck, Milestone Pharmaceuticals, Novartis, Novo Nordisk, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, and participates on clinical trial committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics; Mr. Magaret and Ms. Rhyne are employees of Prevencio, Inc; Dr. Masson is an employee of Roche Diagnostics, Inc; Dr. Butler is a consultant to Abbott, American Regent, Amgen, Applied Therapeutic, AskBio, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardiac Dimension, Cardiocell, Cardior, CSL Bearing, CVRx, Cytokinetics, Daxor, Edwards, Element Science, Faraday, Foundry, G3P, Innolife, Impulse Dynamics, Imbria, Inventiva, Ionis, Levator, Lexicon, Lilly, LivaNova, Janssen, Medtronics, Merck, Occlutech, Owkin, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Pharmain, Prolaio, Pulnovo, Regeneron, Renibus, Roche, Salamandra, Salubris, Sanofi, SC Pharma, Secretome, Sequana, SQ Innovation, Tenex, Tricog, Ultromics, Vifor, and Zoll. Dr. Hansen is an employee of Janssen Research & Development. Ms. Liu has no disclosures.
Figures


References
-
- Oshima M, Shimizu M, Yamanouchi M, Toyama T, Hara A, Furuichi K, et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat Rev Nephrol. 2021;17(11):740–50. - PubMed
-
- Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22(Suppl 1):3–15. - PubMed
-
- Neuen BL, Tuttle KR, Vaduganathan M. Accelerated risk-based implementation of guideline-directed medical therapy for type 2 diabetes and chronic kidney disease. Circulation. 2024;149(16):1238–40. - PubMed
-
- Kidney Disease: Improving global outcomes diabetes work G. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5S):S1-S127. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

