Death-ision: the link between cellular resilience and cancer resistance to treatments
- PMID: 40375296
- PMCID: PMC12080166
- DOI: 10.1186/s12943-025-02339-1
Death-ision: the link between cellular resilience and cancer resistance to treatments
Abstract
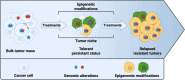
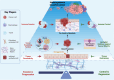
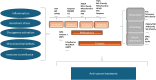
One of the key challenges in defeating advanced tumors is the ability of cancer cells to evade the selective pressure imposed by chemotherapy, targeted therapies, immunotherapy and cellular therapies. Both genetic and epigenetic alterations contribute to the development of resistance, allowing cancer cells to survive initially effective treatments. In this narration, we explore how genetic and epigenetic regulatory mechanisms influence the state of tumor cells and their responsiveness to different therapeutic strategies. We further propose that an altered balance between cell growth and cell death is a fundamental driver of drug resistance. Cell death programs exist in various forms, shaped by cell type, triggering factors, and microenvironmental conditions. These processes are governed by temporal and spatial constraints and appear to be more heterogeneous than previously understood. To capture the intricate interplay between death-inducing signals and survival mechanisms, we introduce the concept of Death-ision. This framework highlights the dynamic nature of cell death regulation, determining whether specific cancer cell clones evade or succumb to therapy. Building on this understanding offers promising strategies to counteract resistant clones and enhance therapeutic efficacy. For instance, combining DNMT inhibitors with immune checkpoint blockade may counteract YAP1-driven resistance or the use of transcriptional CDK inhibitors could prevent or overcome chemotherapy resistance. Death-ision aims to provide a deeper understanding of the diversity and evolution of cell death programs, not only at diagnosis but also throughout disease progression and treatment adaptation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Surmounting cancer drug resistance: New insights from the perspective of N6-methyladenosine RNA modification.Drug Resist Updat. 2020 Dec;53:100720. doi: 10.1016/j.drup.2020.100720. Epub 2020 Aug 20. Drug Resist Updat. 2020. PMID: 32892147 Review.
-
Stem cell programs in cancer initiation, progression, and therapy resistance.Theranostics. 2020 Jul 9;10(19):8721-8743. doi: 10.7150/thno.41648. eCollection 2020. Theranostics. 2020. PMID: 32754274 Free PMC article. Review.
-
Insights into new mechanisms and models of cancer stem cell multidrug resistance.Semin Cancer Biol. 2020 Feb;60:166-180. doi: 10.1016/j.semcancer.2019.07.022. Epub 2019 Jul 29. Semin Cancer Biol. 2020. PMID: 31369817 Review.
-
The impact of non-genetic heterogeneity on cancer cell death.Crit Rev Biochem Mol Biol. 2018 Feb;53(1):99-114. doi: 10.1080/10409238.2017.1412395. Epub 2017 Dec 18. Crit Rev Biochem Mol Biol. 2018. PMID: 29250983 Free PMC article. Review.
-
Mathematical models of cell phenotype regulation and reprogramming: Make cancer cells sensitive again!Biochim Biophys Acta Rev Cancer. 2017 Apr;1867(2):167-175. doi: 10.1016/j.bbcan.2017.04.001. Epub 2017 Apr 7. Biochim Biophys Acta Rev Cancer. 2017. PMID: 28396217 Free PMC article. Review.
References
-
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. - PubMed
-
- Lee-Six H, Olafsson S, Ellis P, Osborne RJ, Sanders MA, Moore L, et al. The landscape of somatic mutation in normal colorectal epithelial cells. Nature. 2019;574:532–7. - PubMed
-
- Weeden CE, Hill W, Lim EL, Grönroos E, Swanton C. Impact of risk factors on early cancer evolution. Cell. 2023;186:1541–63. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

